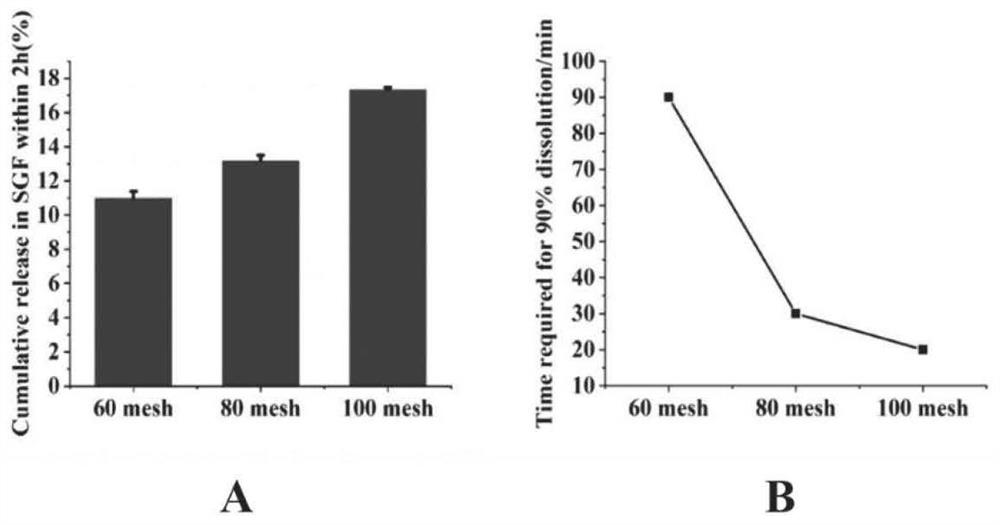
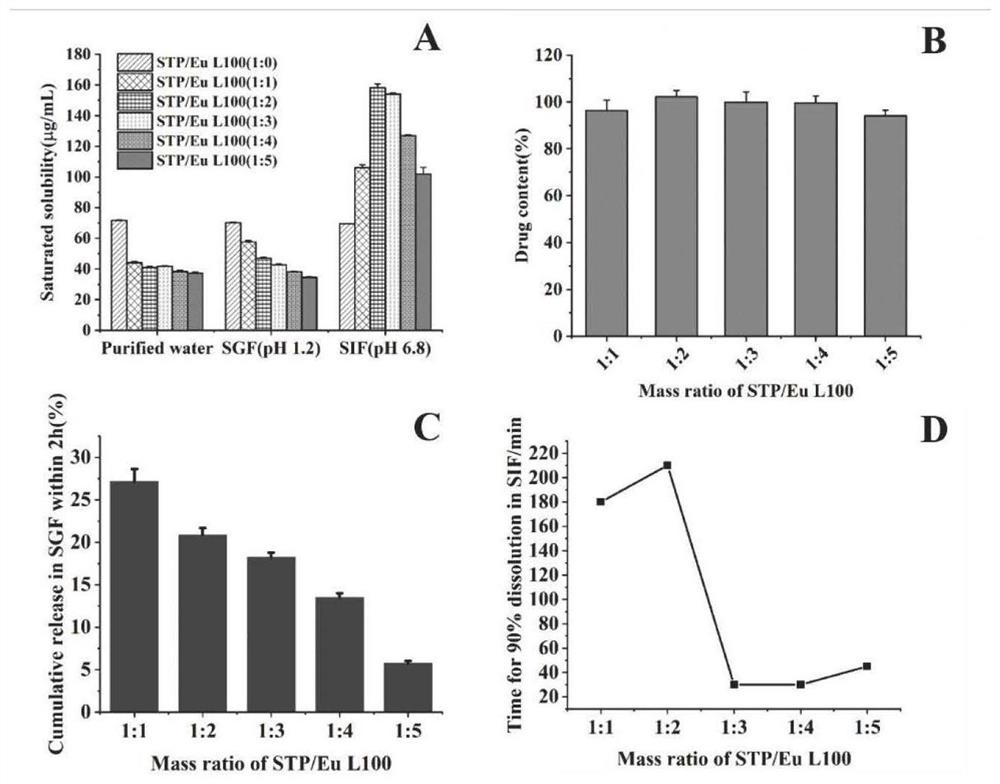
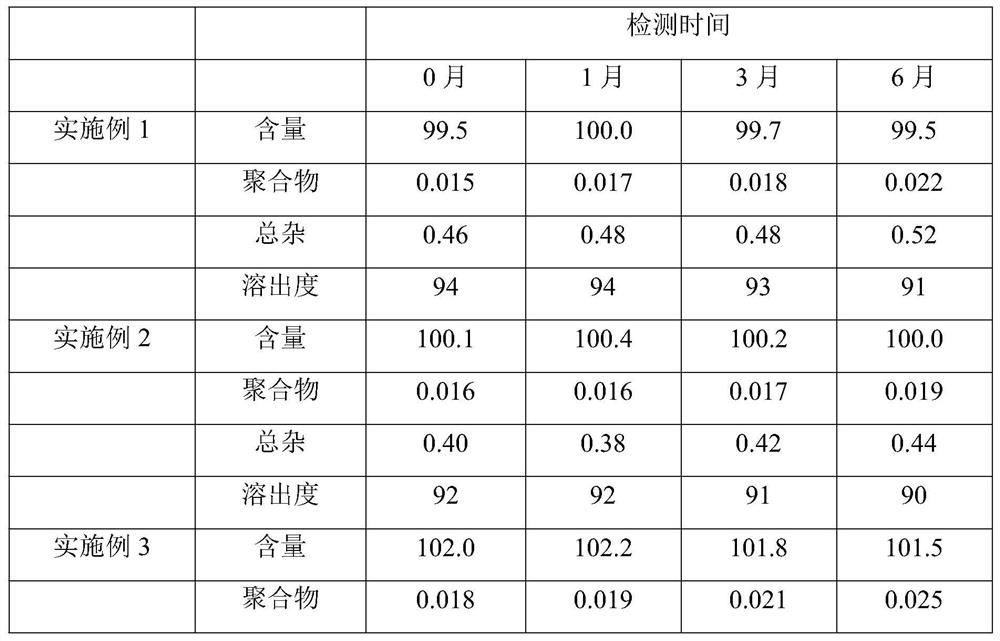
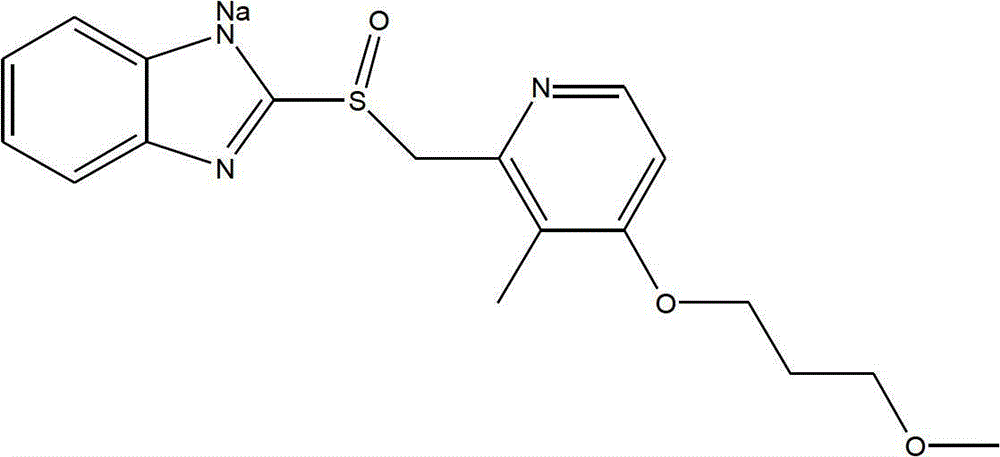
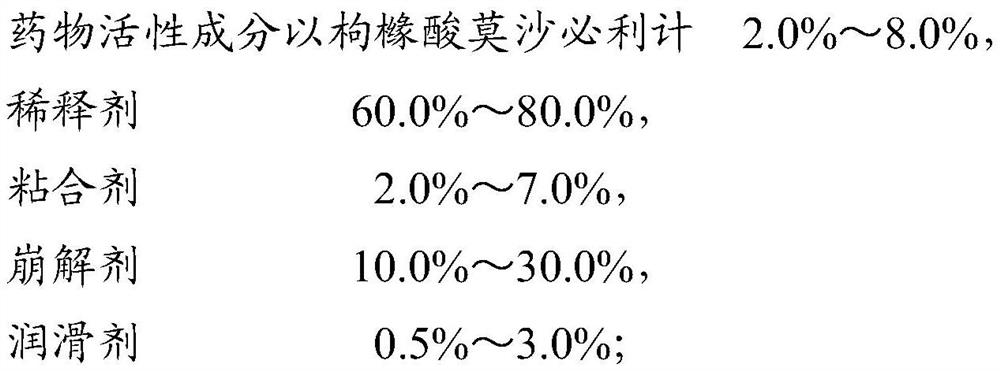
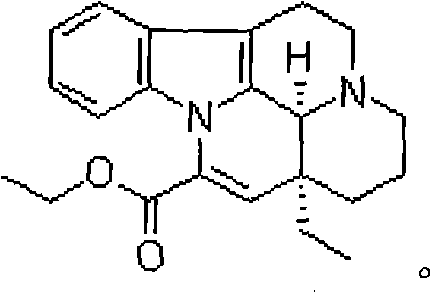
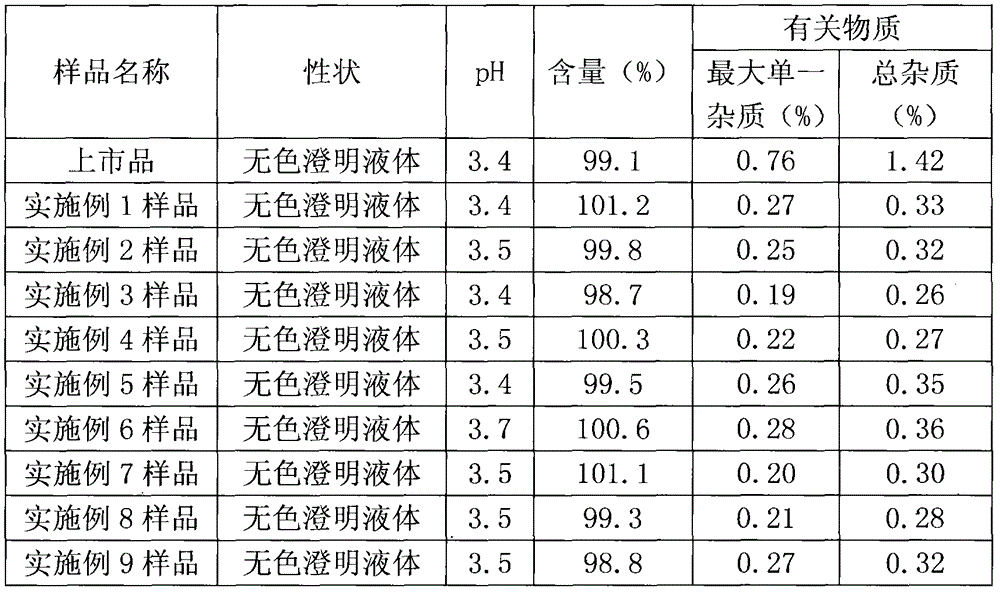
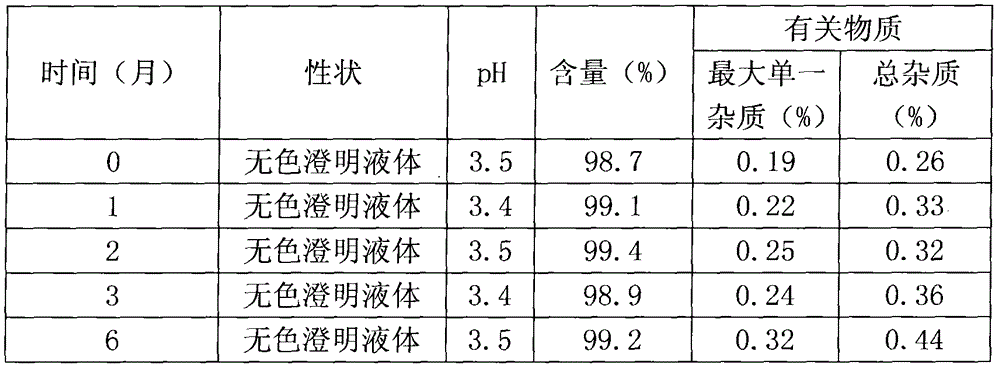
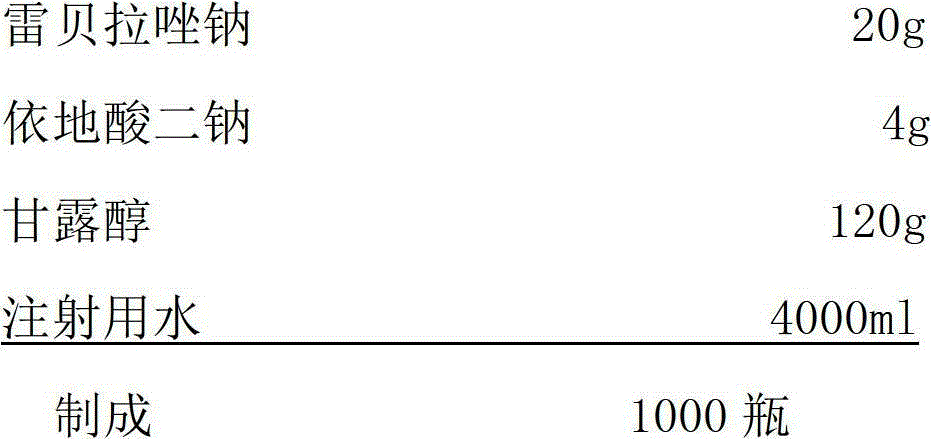Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36results about How to "The composition of the prescription is simple" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Amoxicillin pharmaceutical preparation and preparation method thereof
ActiveCN107875136APromote dissolutionThe composition of the prescription is simpleAntibacterial agentsPharmaceutical non-active ingredientsMagnesium stearateAmoxicillin
The invention discloses an amoxicillin pharmaceutical preparation and a preparation method thereof. The amoxicillin pharmaceutical preparation comprises the following components in parts by mass: 90.0-99.9 parts of amoxicillin and 0.1-10.0 parts of magnesium stearate. The preparation method is a dry-press process. By controlling the particle size distribution of amoxicillin, the particle size distribution of magnesium stearate and the pressure of the dry press, excellent dissolution quantity and stability of the amoxicillin preparation can be ensured.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Vancomycin hydrochloride for injection and its preparing method
InactiveCN1857716AThe composition of the prescription is simpleThe method is reliable and practicalAntibacterial agentsPharmaceutical delivery mechanismVancomycin HydrochlorideGlycine
The vancomycin hydrochloride injection consists of medicine vancomycin hydrochloride in 90%-99% and supplementary material in 1%-10%, and is prepared into powder for injection with supplementary material of stabilizer and / or antioxidant. The medicine powder has potency higher than 900 IU / mg, and is dissolved in water to form 50 mg / ml solution with absorbance at 465 nm lower than 0.065 and pH 2.0-4.0. It is prepared through freeze drying process. The vancomycin hydrochloride injection of the present invention has simple preparation process, common medicinal supplementary material citric acid, glycine, L-cysteine etc, and high normal temperature storage stability.
Owner:ZHEJIANG UNIV +1
Myricetin nanosuspension and preparation method thereof
InactiveCN103083235AAvoid destructionImprove propertiesAntibacterial agentsOrganic active ingredientsSolubilityPolymer science
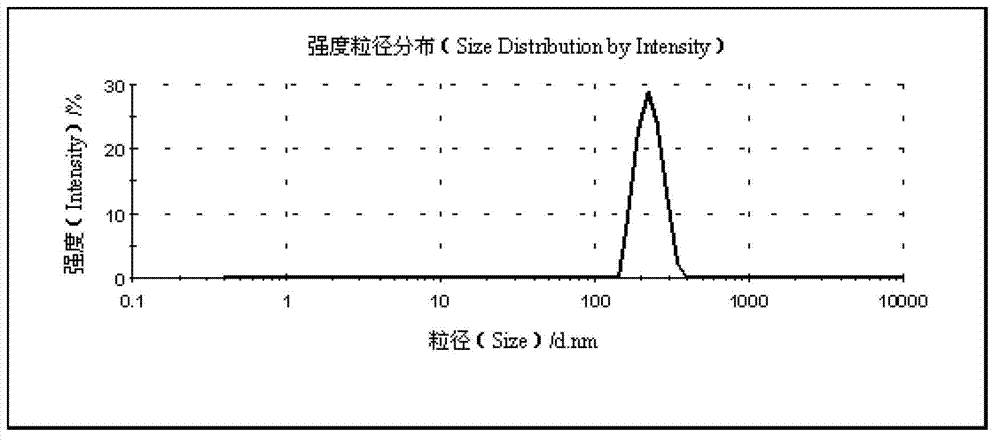
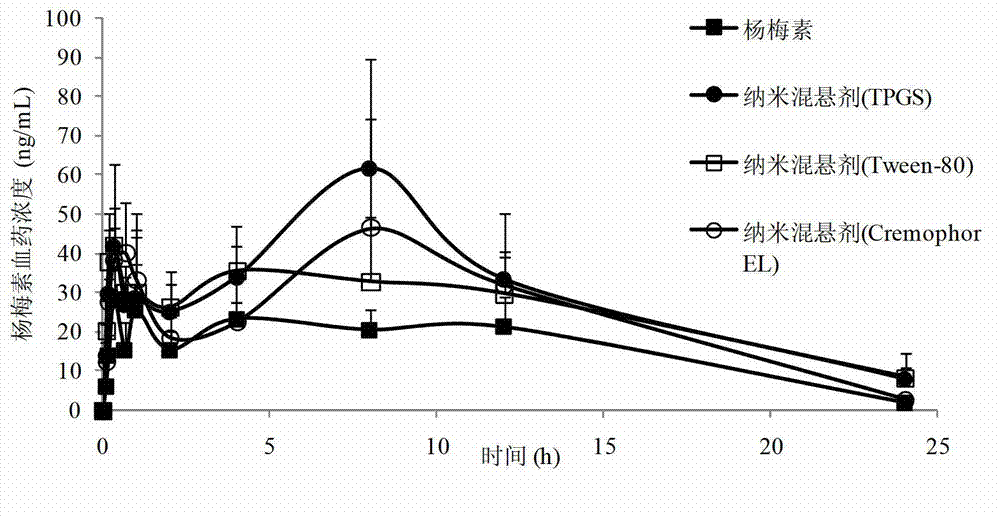
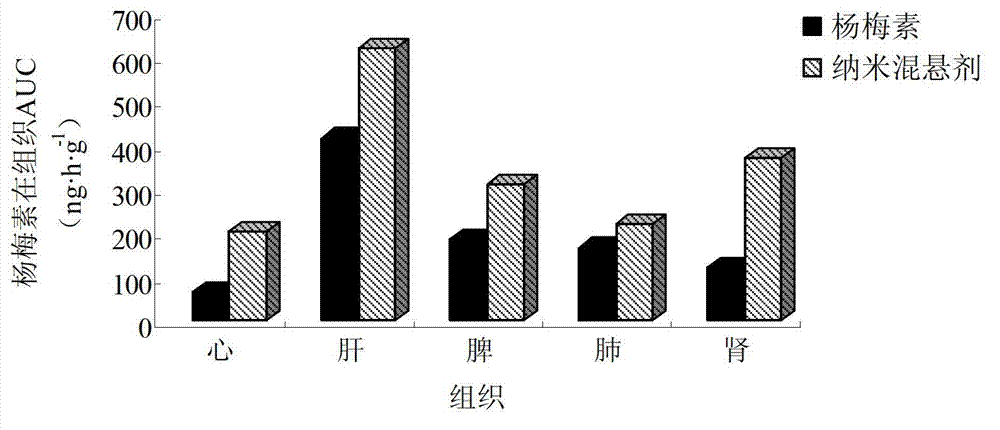
The invention belongs to the technical field of medicines and relates to a myricetin nanosuspension and a preparation method thereof. The myricetin nanosuspension is prepared by adopting a settling method and a high-pressure homogenization method, and the formula comprises myricetin and a stabilizer according to the weight ratio of 1: 0.25-1: 2.5. According to the preparation method provided by the invention, the formula is optimized, Tween-80, Cremophor EL and D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) are screened as the stabilizer, the prepared myricetin nanosuspension has stable nature and simple composition of the formula, the preparation process is simple, convenient and feasible, and the particle size range is 100nm-300nm; and by reducing the particle size of a medicament, the solubility and the dissolution of the myricetin are obviously improved, and the oral bioavailability and the in-vivo tissue distribution of the myricetin are further improved. In addition, the myricetin nanosuspension can be freeze-dried, and proper excipients are added into the obtained freeze-dried powder for further preparing oral liquid, tablets, granules, capsules and other different oral preparations so as to facilitate clinical applications.
Owner:SHANGHAI UNIV OF T C M
Medical composition
ActiveCN102038680AImprove stabilityImprove product qualityOrganic active ingredientsNervous disorderMedicineAir quality index
Owner:常建晖
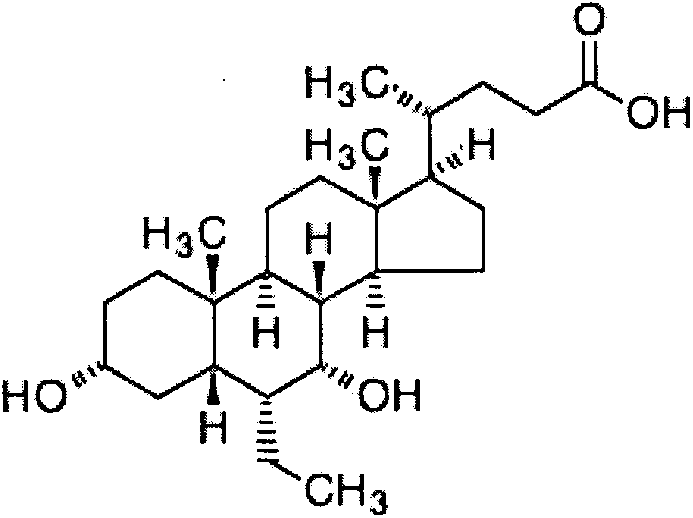
Oral disintegrating tablet of obeticholic acid, and preparation method thereof
InactiveCN105997909AThe composition of the prescription is simplePromote dissolutionOrganic active ingredientsDigestive systemActive componentBULK ACTIVE INGREDIENT
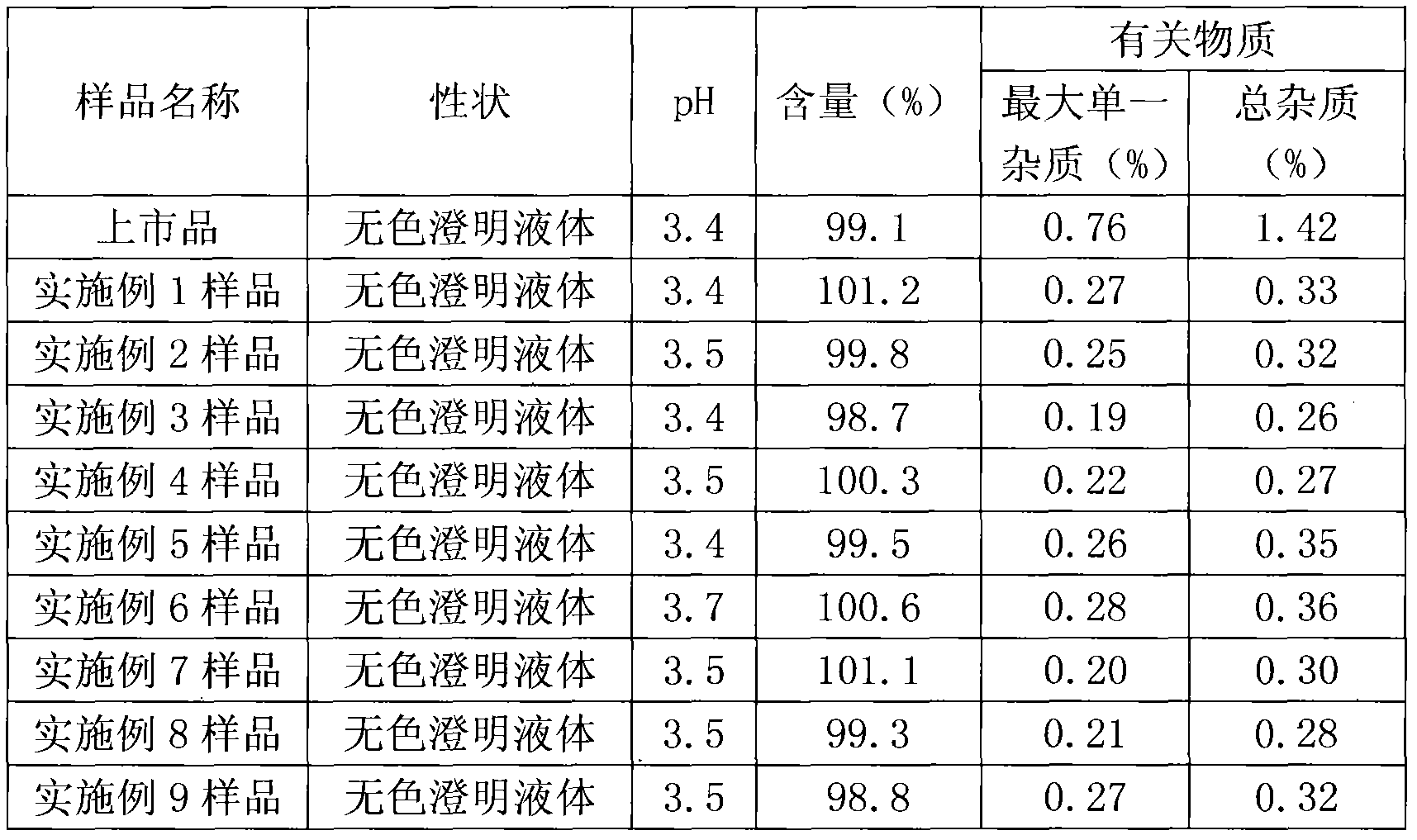
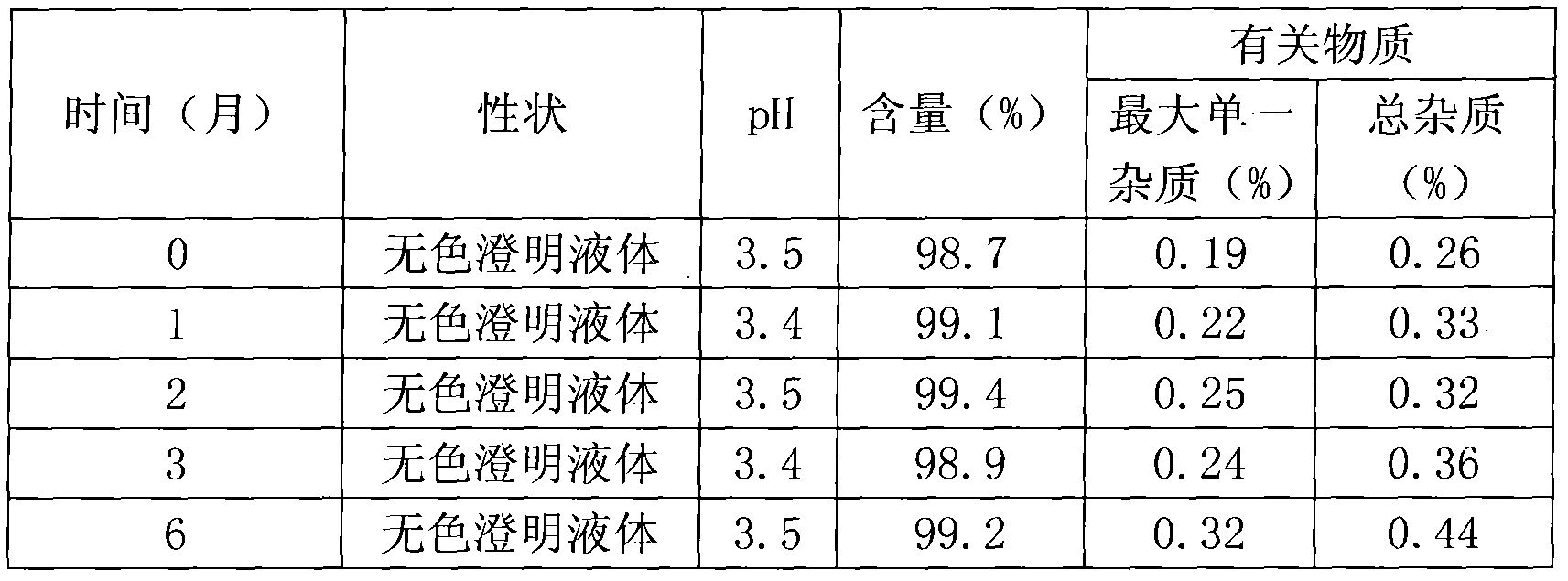
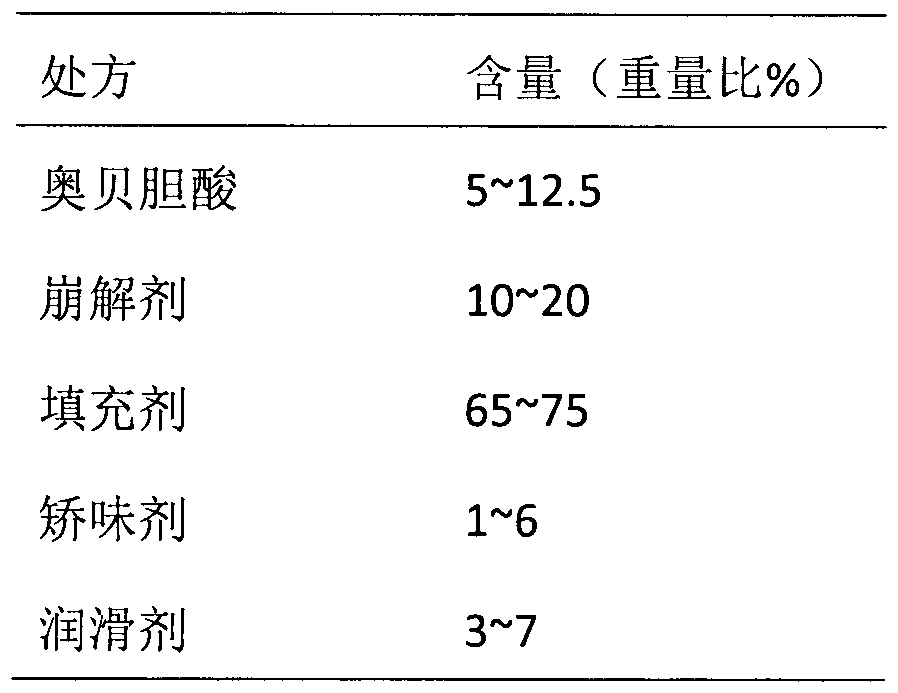
The invention belongs to the field of pharmaceutical preparations, and in particular relates to an orally disintegrating obeticholic acid tablet and a preparation method thereof. The orally disintegrating tablet of obeticholic acid of the present invention is composed of active ingredient obeticholic acid, filler, disintegrant, flavoring agent and lubricant, and its weight percentage is: 5‑12.5: 65‑75: 10‑20 : 1-6: 3-7. The invention adopts a powder direct tableting method to prepare obeticholic acid orally disintegrating tablets, and the process is simple and suitable for large-scale industrial production. The disintegration time, content uniformity, stability and the like of the obeticholic acid orally disintegrating tablet prepared by the present invention all meet the pharmacopoeia standards.
Owner:CHINA PHARM UNIV
Huperzine osmotic-pump controlled release tablet
InactiveCN102512395AProlong the action timeStable effective therapeutic concentrationOrganic active ingredientsNervous disorderCellulose acetateSuspending Agents
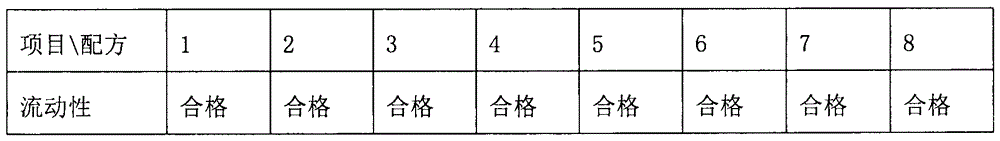
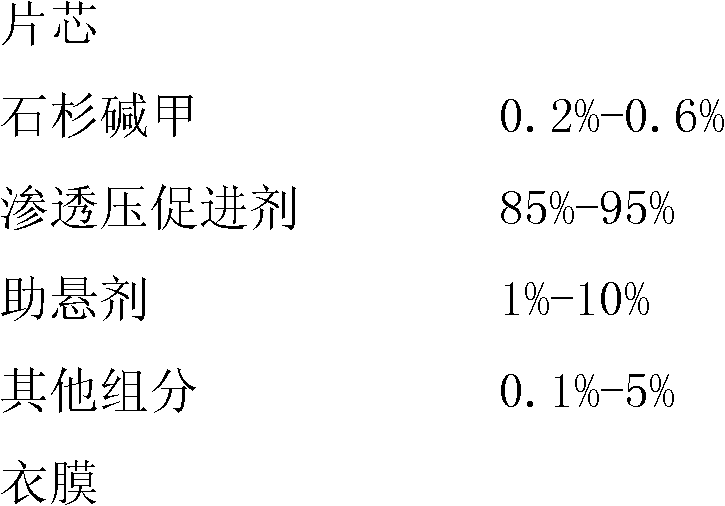
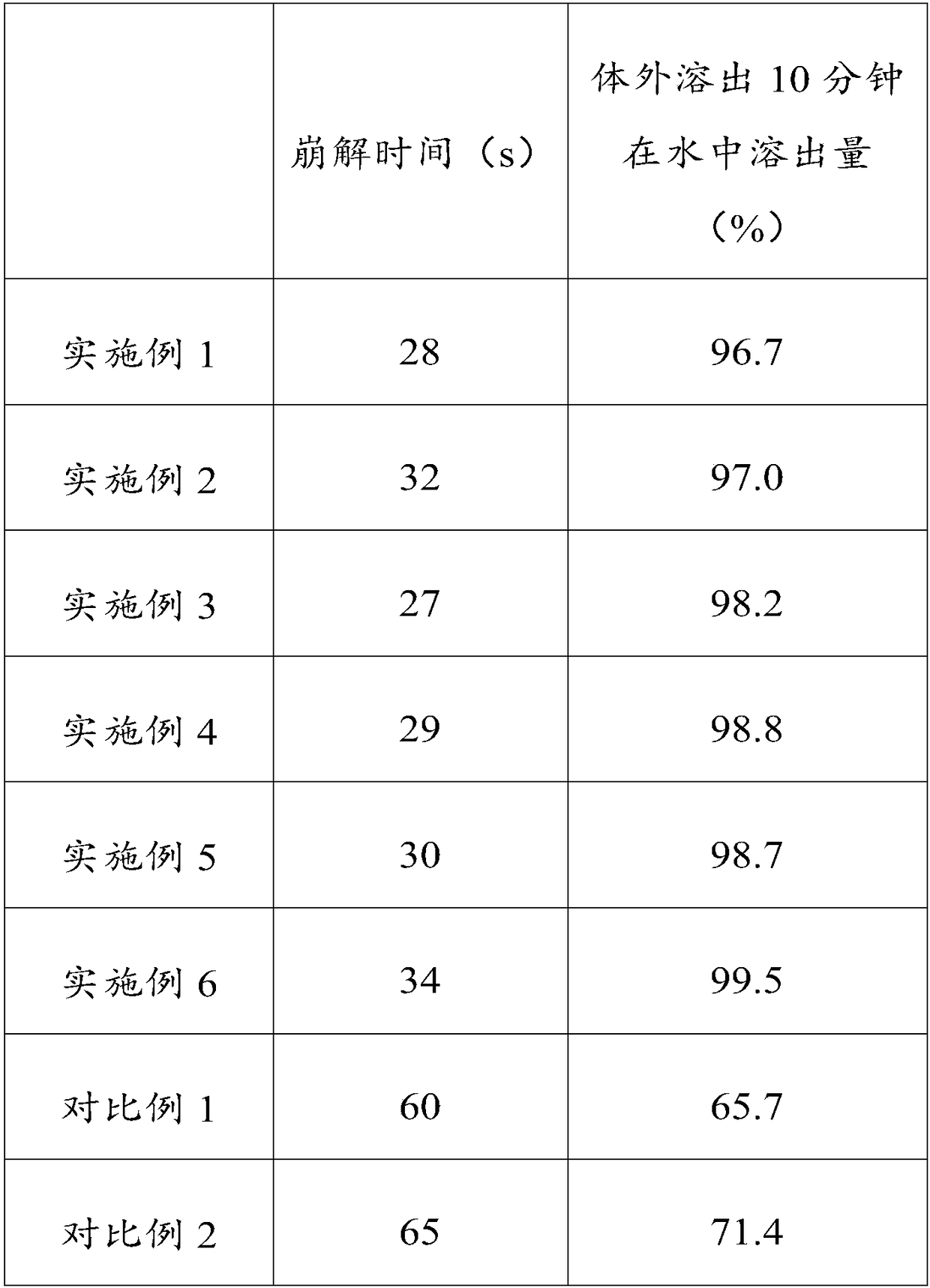
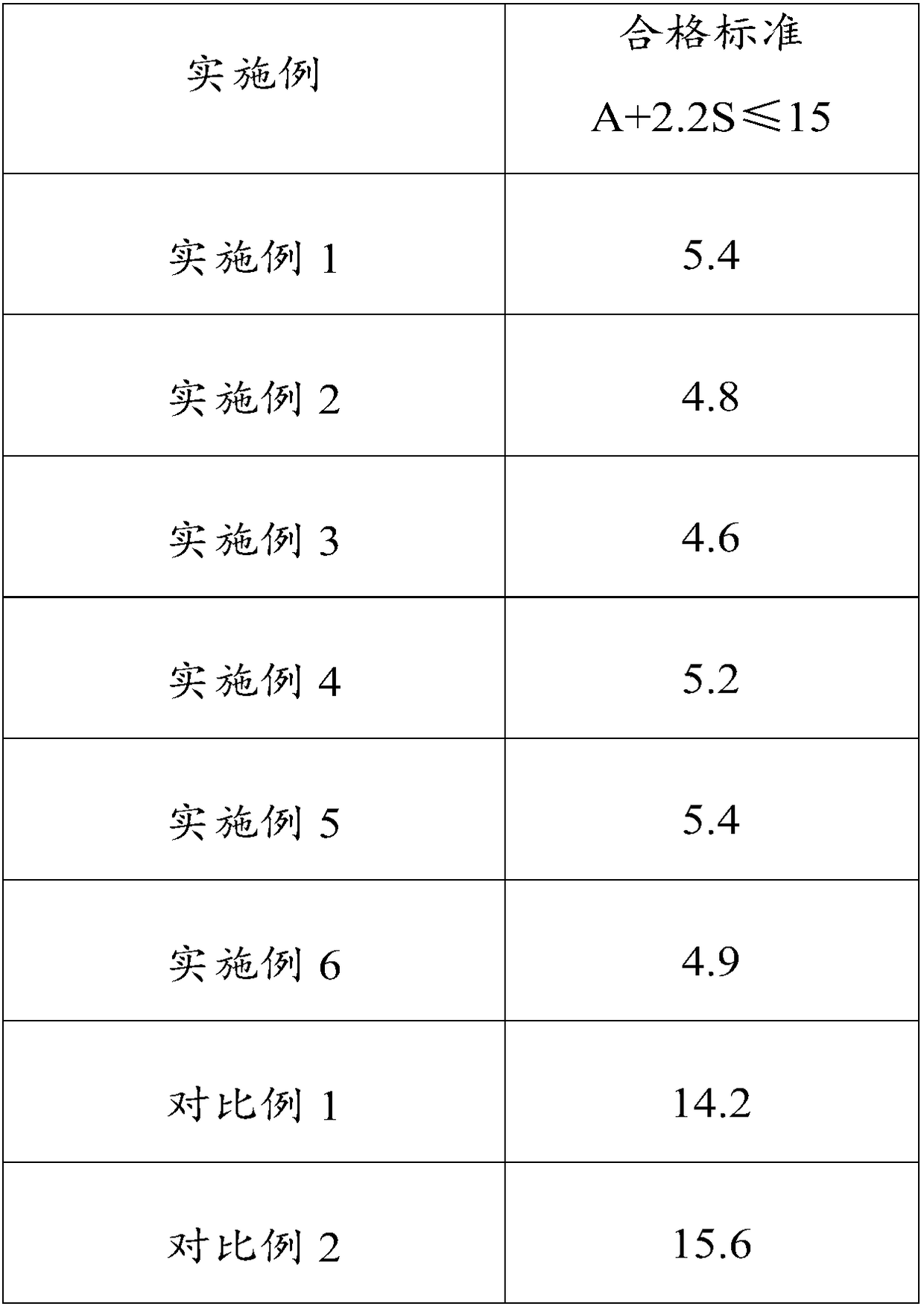
The invention relates to a huperzine osmotic-pump controlled release tablet, which is composed of tablet core containing huperzine and semipermeable film coating with small holes. The huperzine osmotic-pump controlled release preparation is characterized in that the preparation comprises the following components by the weight percent: 0.2-0.6 percent of huperzine in the tablet core, 85-95 percent of osmotic pressure accelerating agent, 1-10 percent of suspending agent and o.1-5 percent of other components. A coating film contains cellulose acetate, PEG (polyethyleneglycol) 4000 and a plasticizing agent, and the proportion of the cellulose acetate, the PEG4000 and the plasticizing agent is 1: (0.3-0.4) : (0.1-0.2). The weight of the coating is increased by 11-13 percent.
Owner:北京振东生物科技有限公司
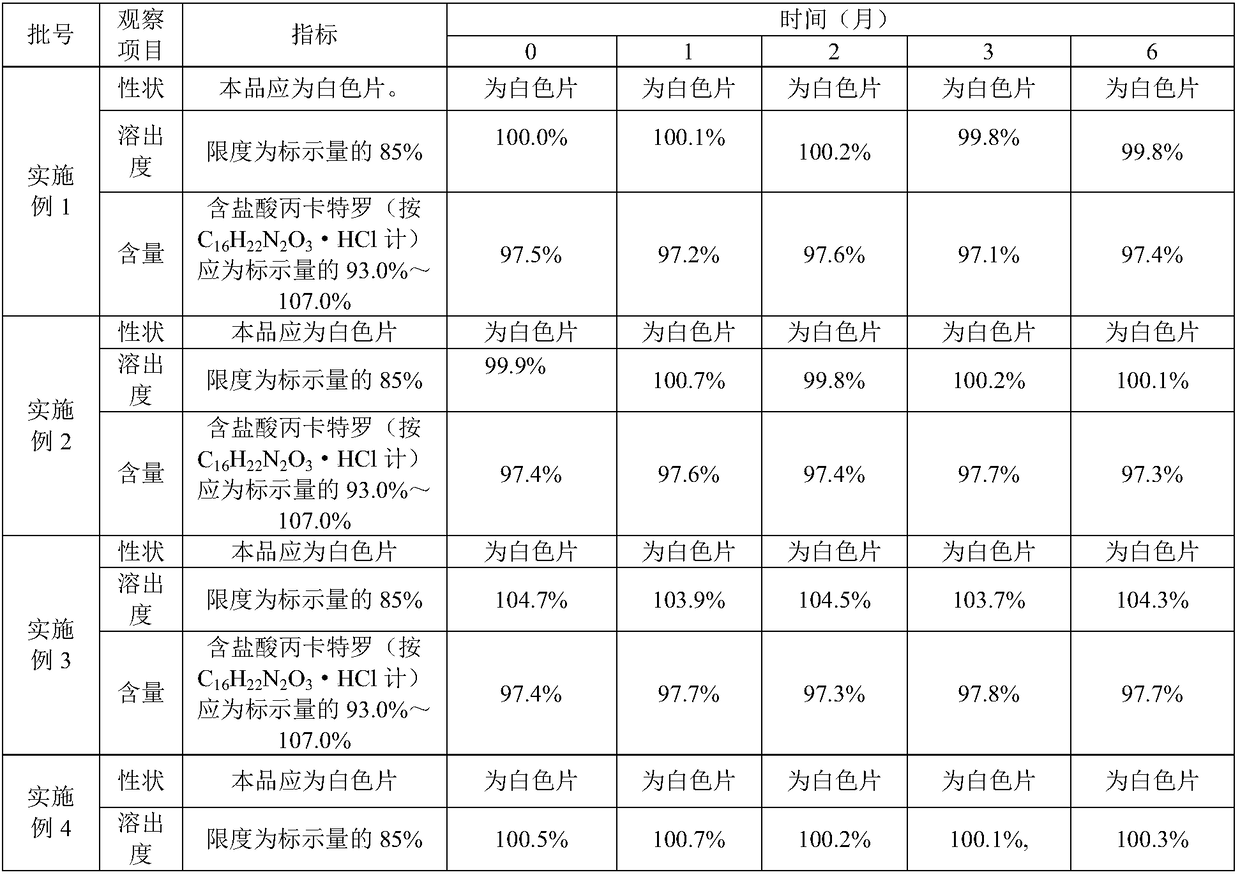
Procaterol hydrochloride tablets and preparation method thereof
InactiveCN108295035AGood disintegrationDissolution rate is fastOrganic active ingredientsPill deliveryFiller ExcipientMedical prescription
The invention relates to the field of pharmacy, in particular to procaterol hydrochloride tablets and a preparation method thereof. The procaterol hydrochloride tablets are prepared from a plurality of raw and auxiliary materials: 0.002 to 0.003 part of procaterol hydrochloride, 0.1 to 0.2 part of a disintegrating agent, 1 to 2 parts of a first filling agent and 3.5 to 5 parts of auxiliary materials. According to the preparation method of the procaterol hydrochloride tablets, various raw and auxiliary materials can be fully and uniformly mixed; the yield is high; the main components of the tablets are not wrapped by the auxiliary materials, so that the dissolving-out speed is high, and the stability is high. Meanwhile, the tablets are low in dose and simple in prescription, and only contain the several raw and auxiliary materials; the energy consumption in a preparation process is low.
Owner:重庆希尔安药业有限公司
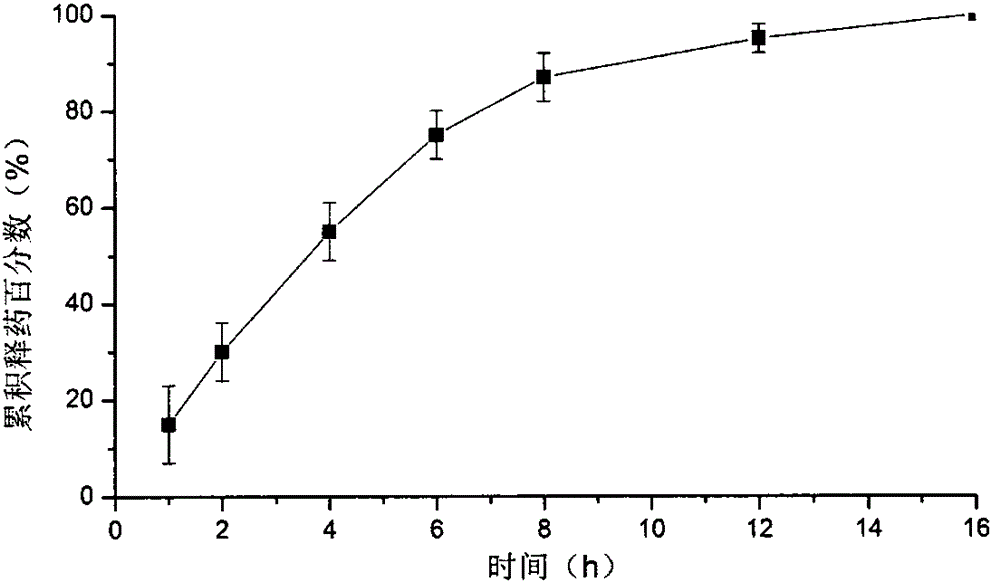
Indissolvable drug oral sustained-release dry emulsion tablet and preparation method thereof
InactiveCN105168163ADissolution medium volume is smallIncrease speedOrganic active ingredientsPharmaceutical delivery mechanismBlood concentrationDissolution
The invention belongs to the field of medicinal preparations, and discloses an indissolvable drug oral sustained-release dry emulsion tablet and a preparation method thereof. The preparation method for the oral sustained-release dry emulsion tablet comprises the steps that after an oil phase, a surface active agent and cosurfactant are evenly mixed, an indissolvable drug is added till the drug is dissolved completely, and accordingly a medicine-carried self-emulsifying system is prepared; after the medicine-carried self-emulsifying system and an aqueous solution of a hydrophilic gel framework material are evenly mixed, spray drying is conducted, and sustained-release dry emulsion powder is obtained; after the sustained-release dry emulsion powder and a conventional tablet auxiliary material needed for preparation are mixed, wet granulation is carried out; at last, tabletting is performed. The sustained-release dry emulsion tablet can improve the dissolution and dissolving-out performance of the indissolvable drug, the bioavailability of the indissolvable drug is improved, a stable blood concentration is formed, and the compliance of patients is improved. The sustained-release dry emulsion tablet is not complex in composition of prescription, and the preparation technology is suitable for industrial production.
Owner:CHINA PHARM UNIV
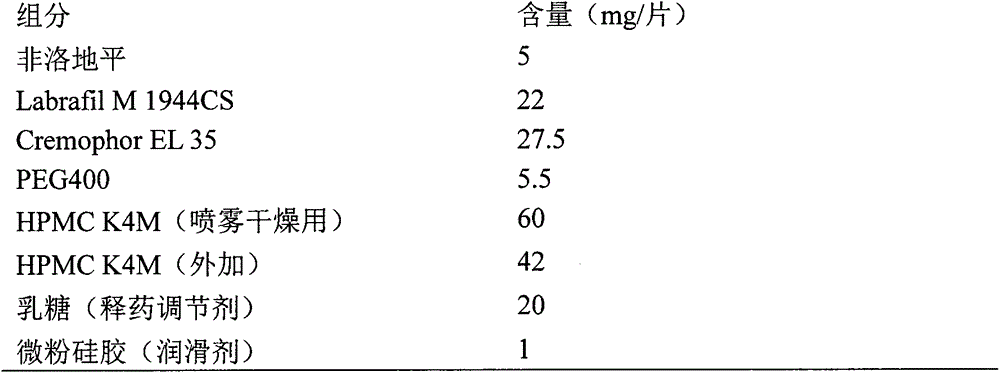
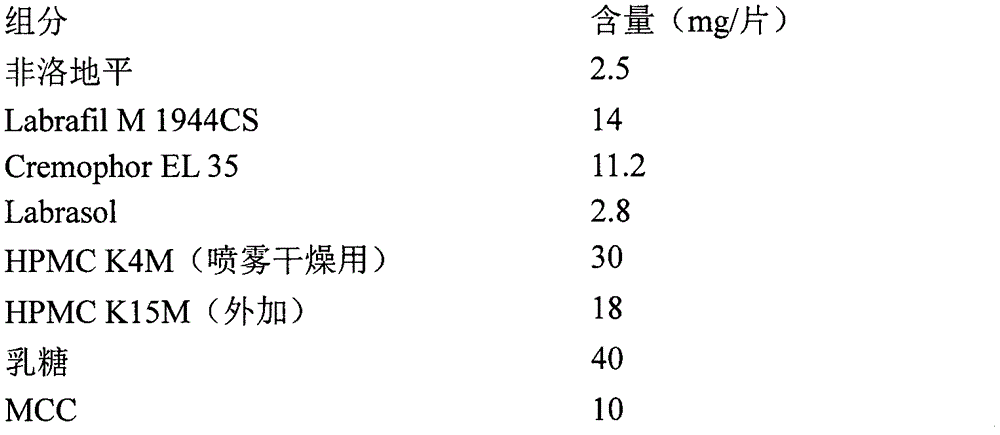
Nifedipine A crystal bulk crystal habit and controlled release tablet composition thereof
ActiveCN112592311AThe composition of the prescription is simpleSimple processOrganic active ingredientsOrganic chemistry methodsControlled Release TabletBulk crystal
The present invention relates to a nifedipine A crystal bulk crystal habit and a controlled release tablet composition thereof, the controlled release tablet composition contains (HPMC) E30 and the nifedipine A crystal habit, the nifedipine A crystal form is cubic, the length, width and thickness ratio is 4-1: 2-1: 1, the particle size range Dv (90) is 120-240 [mu] m, and the mass ratio of HPMC E30 to nifedipine is 2:1 to 3:1. The invention provides the nifedipine bulk crystal habit and a pharmaceutical composition of nifedipine controlled release tablets released at an approximately constantrate.
Owner:DISHA PHARMA GRP
Oral cavity instant membrane agent containing rizatriptan benzoate and preparation method thereof
ActiveCN109730978AFast in vitro dissolution rateLarge specific surface areaOrganic active ingredientsNervous disorderFiberPorosity
The invention belongs to the technical field of medicines, and relates to an oral cavity instant membrane agent containing rizatriptan benzoate prepared by using an electrostatic spinning technology.The formula of the oral cavity instant membrane agent comprises, 1 part of the rizatriptan benzoate, 1-300 parts of a membrane forming material and 0-50 parts of other auxiliaries. An oral cavity instant membrane is obtained through the electrostatic spinning technology, the electrostatic spinning conditions are that the voltage is 5-20 kV, the proceeding speed is 0.1-10 ml / h, the receiving distance is 5-20 cm, the temperature is 5-60 DEG C, and the humidity is 10-70%. The oral cavity instant membrane agent is composed of nano polymer fibers formed by electrostatic spinning, and the diameter of the nano polymer fibers is 10-1000 nm. The rizatriptan benzoate is uniformly distributed without a fixed form state. The oral cavity instant membrane agent containing the rizatriptan benzoate has higher specific surface area and porosity and faster dissolution rate, the effect comes up faster, the biological utilization rate is higher, and the pain of a patient with migraine can be rapidly relieved.
Owner:SHENYANG PHARMA UNIVERSITY
Application of berberine-oryzanol tablet in treatment of diabetes
ActiveCN111686084AIncrease the areaGood dispersionOrganic active ingredientsMetabolism disorderDiabetes mellitusAdjuvant
The invention provides a berberine-oryzanol tablet and application thereof in treatment of diabetes. The berberine-oryzanol tablet is obtained through mixing of modified berberine-oryzanol granules and other adjuvants at a certain ratio and tabletting of the mixture, wherein the modified granules comprise drugs, surfactants and dispersants. Through the modified granules, the solubility of berberine and oryzanol can be improved well, and the dissolution and bioavailability of the drugs can be improved. According to the tablet, a good treatment effect on diabetes can be achieved through compatibility of the drugs, a reasonable prescription and preparation process.
Owner:YICHUN WANSHEN PHARMA MACHINERY
Application of beta-glucan as adhesive in preparation of tablets or granules
ActiveCN111603452AThe composition of the prescription is simpleImprove dispersionPill deliveryPharmaceutical non-active ingredientsGlucanActive agent
The invention relates to the field of pharmaceutical preparations, and provides an application of beta-glucan as an adhesive in the preparation of tablets or granules. Beta-glucan has certain viscosity, can play a certain role in adhesion in the tablet forming process, and can also ensure the dissolution of medicines. The invention further provides a pharmaceutical composition containing beta-glucan. Beta-glucan and a surfactant namely sodium dodecyl sulfate are jointly dispersed in an organic solvent, drying is carried out after sufficient stirring, airflow crushing is further carried out toobtain beta-glucan-sodium dodecyl sulfate modified powder, the dispersity of beta-glucan and the content uniformity of a preparation are effectively improved, and the medicine bioavailability is improved.
Owner:YICHUN WANSHEN PHARMA MACHINERY
Effervescent tablets containing stiripentol solid dispersion and preparation method thereof
ActiveCN113813234AEasy to acceptThere are no problems such as easy cakingPowder deliveryNervous disorderEffervescent tabletSmall intestine
The invention belongs to the field of pharmaceutical preparations, and particularly relates to effervescent tablets containing a stiripentol solid dispersion and a preparation method thereof. According to the effervescent tablets disclosed by the invention, in one aspect, an indissolvable drug is prepared into an amorphous solid dispersion, so that the defect that the permeability of gastrointestinal tracts is reduced by other solubilizing means is overcome while the water solubility and the dissolution rate are improved, and the effervescent tablets have relatively high encapsulation efficiency (16.67%-50%); in the other aspect, a common, easily available and safe enteric-coated material is selected as a solid dispersion carrier to ensure that the stiripentol is released in the small intestine in a positioned manner and cannot be dissociated in advance in a physiological solution to cause damage of gastric acid to medicine like other preparation means; and besides, compared with commercially available dosage forms (capsules and dry suspensions), the effervescent tablets overcome the problem that the content is easy to cake, and has the advantages of stable storage, convenience in taking, good taste, particular suitability for epilepsy children who cannot swallow solid preparations, and the like.
Owner:CHINA PHARM UNIV
Heart boosting pulse restoring tablet and its preparation method
InactiveCN1824159AThe composition of the prescription is simpleLess excipientsPill deliveryCardiovascular disorderSolventPalpitations
A Chinese medicine in the form of tablet for nourishing qi and Yin, promoting blood circulation and pulse, heart blood stagnation, cardialgia, palpitation, etc is prepared from 6 Chinese-medicinal materials including ginseng, astragalus root, red sage root, Chuan-xiong rhizome, etc, lubricant and diluent (microcrystalline cellulose) through extracting in water, removing solvent to obtain powder and other conventional steps.
Owner:BEIJING SHENKELIANHUA TECH
A kind of amoxicillin pharmaceutical preparation and preparation method thereof
ActiveCN107875136BPromote dissolutionThe composition of the prescription is simpleAntibacterial agentsPharmaceutical non-active ingredientsMagnesium stearateStearic acid
The invention discloses an amoxicillin pharmaceutical preparation and a preparation method thereof. The amoxicillin pharmaceutical preparation comprises the following components in parts by mass: 90.0-99.9 parts of amoxicillin and 0.1-10.0 parts of magnesium stearate. The preparation method is a dry-press process. By controlling the particle size distribution of amoxicillin, the particle size distribution of magnesium stearate and the pressure of the dry press, excellent dissolution quantity and stability of the amoxicillin preparation can be ensured.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Sodium rabeprazole composition for injection
ActiveCN102949354BIncrease moisture contentAvoid degradationAntibacterial agentsPowder deliveryFreeze-dryingDisodium Edetate
The present invention provides a sodium rabeprazole composition for injection. The composition comprises sodium rabeprazole and disodium edetate, wherein a weight ratio of the sodium rabeprazole to the disodium edentate is 1:0.15-1. The sodium rabeprazole composition does not contain mannitol and other fillers. According to the sodium rabeprazole composition, under a certain premise, freeze drying rate of the product is significantly improved so as to avoid additional sublimation time, such that production efficiency is improved, and production energy consumption is reduced, wherein the premise comprises that a product appearance is ensured and reconstituting property meets injection requirements. In addition, the method is suitable for industrial mass production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
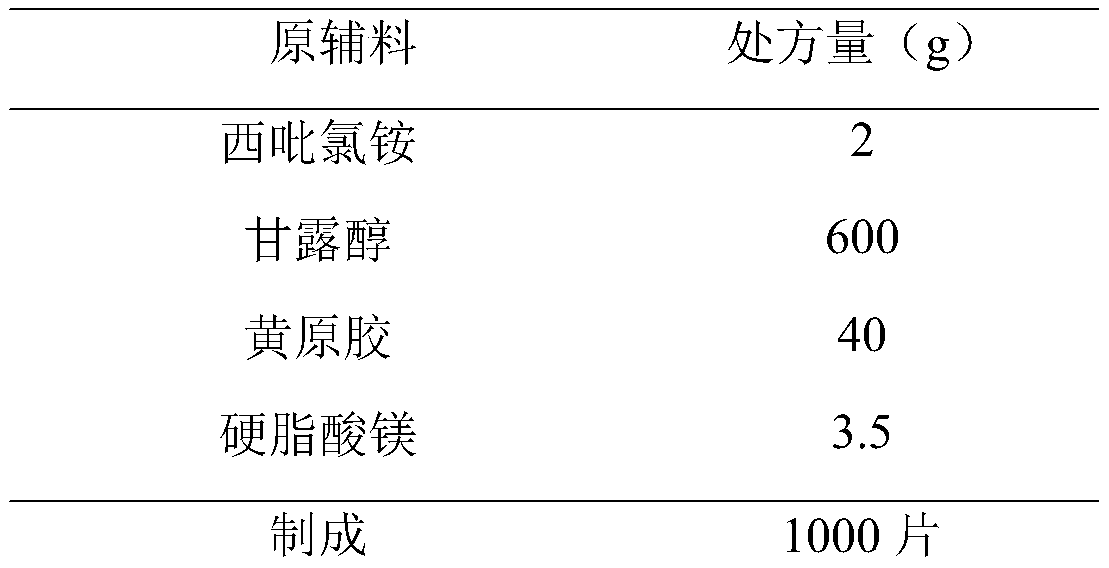
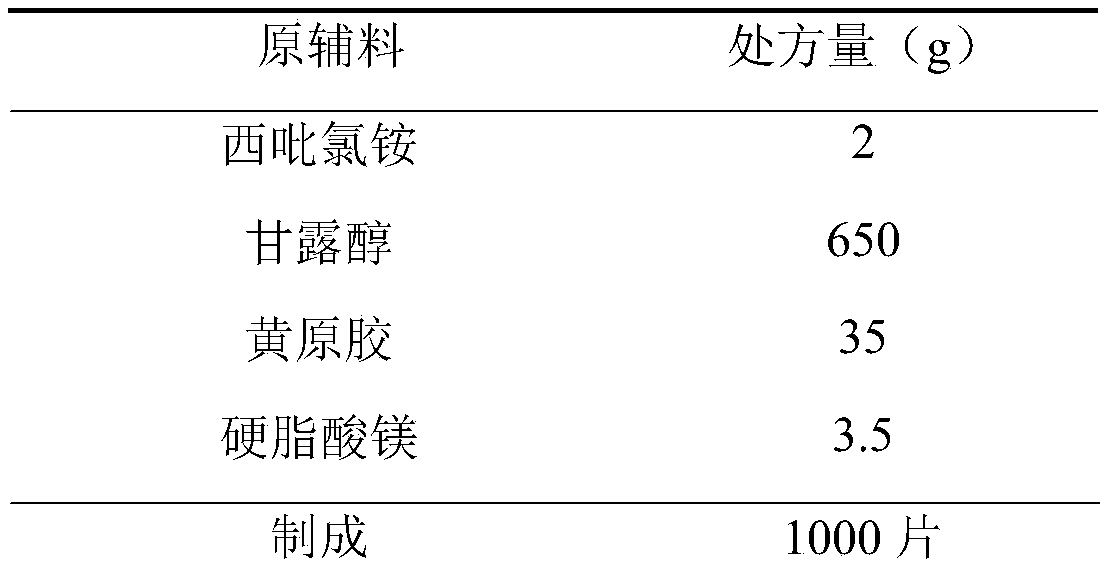
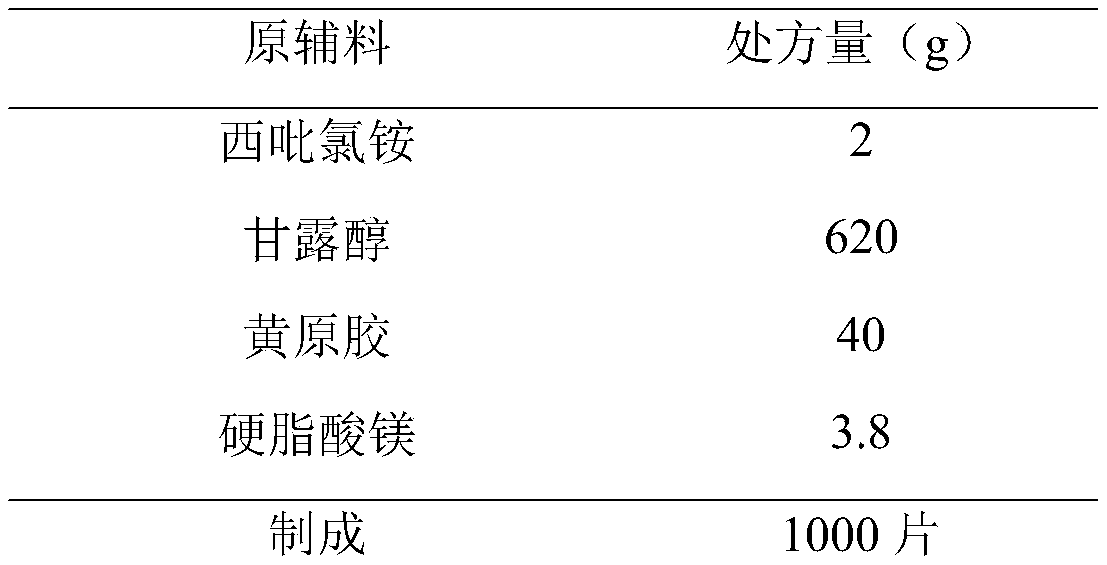
Cetylpyridinium chloride buccal tablet and preparation technology thereof
PendingCN111000809AConvenient sourceThe composition of the prescription is simpleAntibacterial agentsOrganic active ingredientsChemistrySide effect
The invention provides a cetylpyridinium chloride-containing buccal tablet and a preparation technology thereof. The buccal tablet is prepared from cetylpyridinium chloride, xanthan gum, mannitol andmagnesium stearate. The buccal tablet has the advantages of simple prescription composition, great reduction of side effects possibly caused by auxiliary materials, safe and nontoxic auxiliary materials, obvious prolongation of the lasting time of the medicine effect, simple product process, convenience in large-scale production, and safe and reliable product quality. The buccal tablet solves theproblems of medication convenience and compliance of patients.
Owner:四川健能制药有限公司
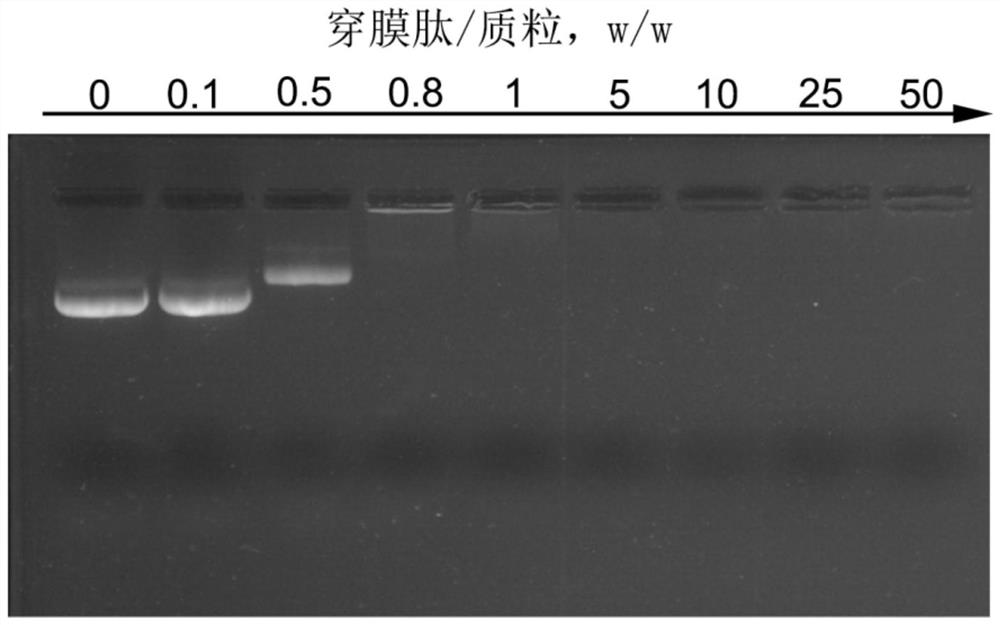
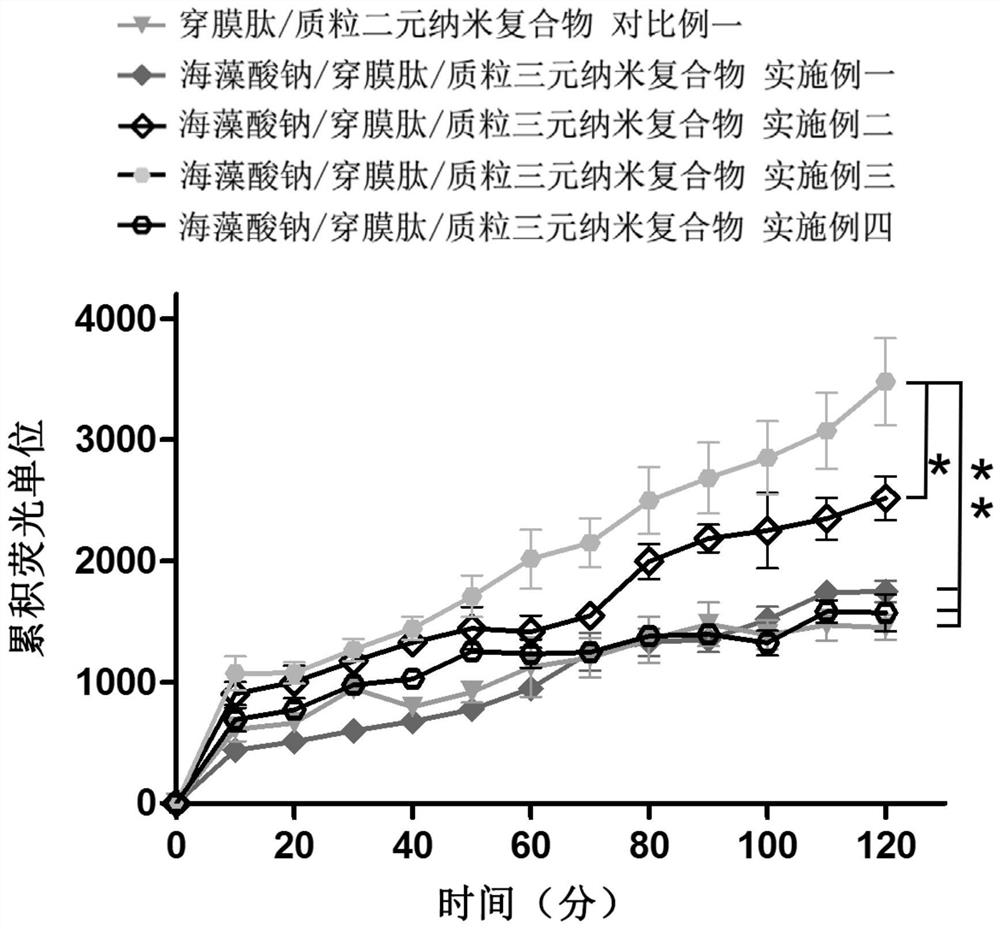
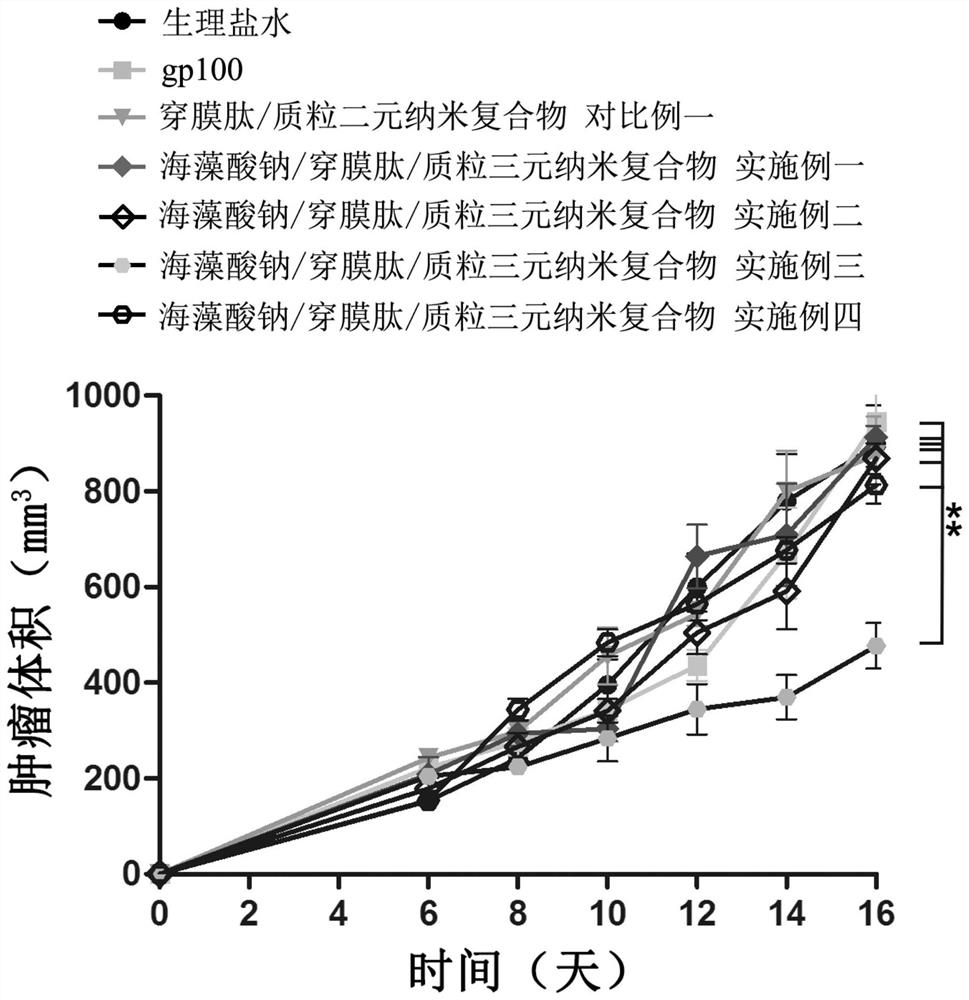
A kind of sodium alginate/penetrating peptide/plasmid ternary nanocomposite vaccine and its preparation and application
ActiveCN110522907BSimple compositionUniform and stable particle sizeCancer antigen ingredientsWhole-cell/virus/DNA/RNA ingredientsMicrobiologyTGE VACCINE
The invention discloses a sodium alginate / penetrating peptide / plasmid ternary nanocomposite vaccine and its preparation and application. The ternary nanocomposite vaccine is composed of sodium alginate, membrane-penetrating peptide and plasmid, wherein the mass ratio of sodium alginate to membrane-penetrating peptide is 1‑5:1, and the mass ratio of membrane-penetrating peptide to plasmid is 5‑400 :1; it can be used to prepare plasmid oral drugs, and provides a new idea for oral delivery of plasmids. The preparation method is to obtain a sodium alginate / membrane penetrating peptide / plasmid ternary nanocomposite vaccine through electrostatic adsorption-mediated layer-by-layer assembly. The preparation conditions are mild, the preparation process is easy to control, the preparation method is simple, and the particle size of the obtained complex is uniform and stable. , is conducive to industrial production.
Owner:ZHEJIANG UNIV
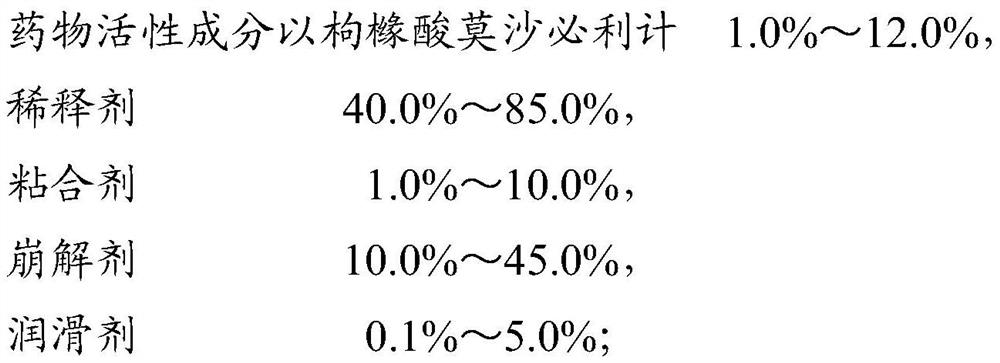
A kind of capsule containing mosapride citrate and preparation method thereof
ActiveCN108324697BRapid dissolutionHigh dissolution rateOrganic active ingredientsDigestive systemMosapride citrateMedicinal chemistry
The invention relates to a capsule containing mosapride citrate and a preparation method thereof. The capsule includes drug granules and a capsule casing. The drug granules contain the mosapride citrate and / or a mosapride citrate dihydrate, a diluent, a binder, a disintegrating agent and a lubricant. The mosapride citrate in the capsule can be quickly dissolved out at high dissolution rate, so that bioavailability is increased. The formula and the preparation method are simple, have high controllability and low production cost, and are easy to achieve in industrial production.
Owner:科贝源(北京)生物医药科技有限公司
A kind of oral preparation containing polypeptide drug self-nanoemulsion and preparation method thereof
ActiveCN106334185BQuality controllableStable in naturePeptide/protein ingredientsMetabolism disorderPeptide drugActive agent
Owner:GUANGDONG GENERAL HOSPITAL
Orally disintegrating tablet containing faropenem sodium and preparation method thereof
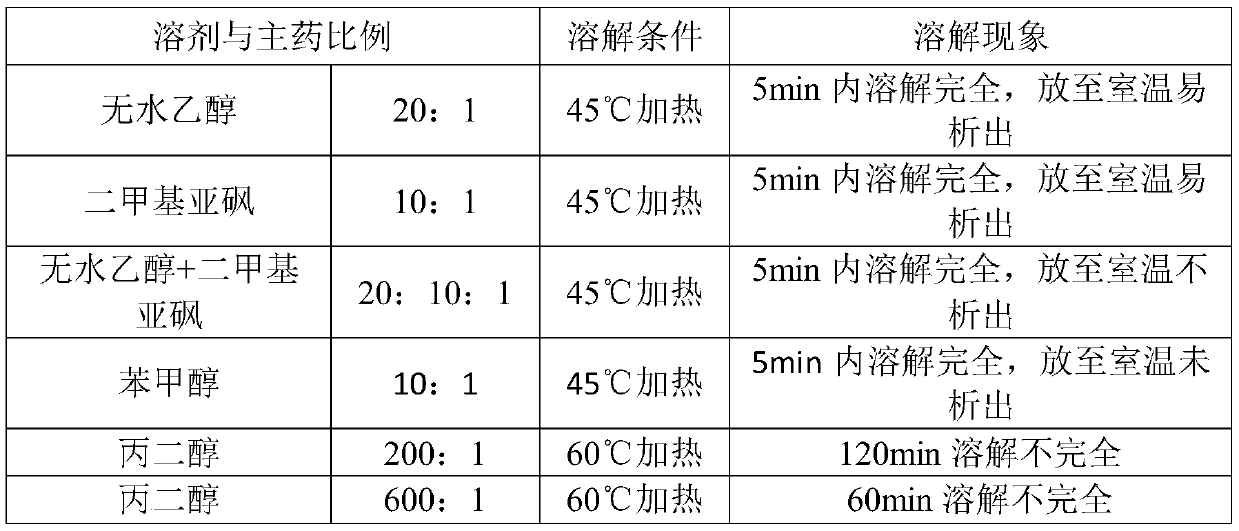
ActiveCN104257618BReduce usageImproved dissolution behaviorPharmaceutical non-active ingredientsPill deliveryMANNITOL/SORBITOLOrally disintegrating tablet
The invention belongs to the field of medicines and provides an orally disintegrating tablet containing faropenem sodium. The orally disintegrating tablet also contains mannitol and a lubricating agent. According to the orally disintegrating tablet, less auxiliary materials are used; the composition formula is simple; the orally disintegrating tablet which is prepared from powder through tabletting still has good fluidity; the obtained tablet is reliable in hardness and high in disintegrating speed; and the dissolution behavior of the main drug is excellent.
Owner:SHANDONG NEWTIME PHARMA
Alchlorine dipropionate containing cream preparation and preparation method thereof
ActiveCN111329834ASolve insolubleImprove stabilityOrganic active ingredientsAntipyreticPropanoic acidOil phase
The invention belongs to the field of pharmaceutical preparations, and particularly relates to an alchlorine dipropionate composition. The composition comprises absolute ethyl alcohol, dimethyl sulfoxide, an oil phase component, a pH regulator, a bacteriostatic agent, a humectant and a metal ion chelating agent. A mixed solvent of absolute ethyl alcohol-dimethyl sulfoxide is adopted as a solvent and a stabilizer of a main drug, the problems that alchlorine dipropionate is difficult to dissolve and unstable in a dissolved state are successfully solved, and the dissolving time of the bulk drug is shortened. In the preparation process of the composition, no special requirement for the particle size of the bulk drug exists, the bulk drug does not need to be subjected to micronization pretreatment, and the preparation process steps are reduced; the prepared alchlorine dipropionate cream is uniform and fine in cream body; and auxiliary materials in the prescription are economical and easy toobtain, the process operation is simple, and industrial mass production is facilitated. The adopted solvent system provides an idea for development of other external dosage forms of alchlorine dipropionate including ointment, lotion and gel.
Owner:CHONGQING HUAPONT PHARMA
A kind of oral instant film containing rizatriptan benzoate and preparation method thereof
ActiveCN109730978BPromotes even distributionWell distributedOrganic active ingredientsNervous disorderBenzoic acidElectrospinning
The invention belongs to the technical field of medicine, and relates to an oral instant film preparation containing rizatriptan benzoate prepared by an electrospinning process. The oral instant film prescription comprises 1 part of rizatriptan benzoate, 1-300 parts of film-forming material and 0-50 parts of other auxiliary materials. Oral instant film is obtained through electrospinning process. The conditions of electrospinning are: voltage 5-20kV, advancing speed 0.1-10ml / h, receiving distance 5-20cm, temperature 5-60℃, humidity 10-70%. The oral instant film is composed of nano-polymer fibers formed by electrospinning, and the diameter of the nano-polymer fibers is 10-1000nm. The rizatriptan benzoate is uniformly distributed in an amorphous state in the film preparation. The oral instant film containing rizatriptan benzoate protected by the present invention has higher specific surface area and porosity and faster dissolution rate, more rapid onset, higher bioavailability, and can quickly relieve migraine patient suffering.
Owner:SHENYANG PHARMA UNIVERSITY
Stable liquid medicinal composition
ActiveCN102526042BImprove stabilityImprove product qualityOrganic active ingredientsNervous disorderPolymer scienceHigh heat
Owner:吉瑞医药(中国)有限公司
A kind of esomeprazole enteric-coated pellets and preparation method thereof
InactiveCN104224728BLow priceWide variety of sourcesOrganic active ingredientsDigestive systemSide effectMagnesium salt
The invention discloses an enteric-coated micropill preparation of esomeprazole and a preparation method thereof, which is prepared by coating the drug-containing micropill with an isolation coat and an enteric-coated coating; Meprazole active ingredient, crystal form stabilizer, basifying agent; the active ingredient of esomeprazole is the crystalline hydrate of esomeprazole strontium salt or magnesium salt; the crystal form stabilizer is polyoxyethylene A mixture of PEO and hypromellose or a mixture of polyoxyethylene PEO and polyvinylpyrrolidone; the weight ratio of the esomeprazole active ingredient, crystal form stabilizer and alkalizing agent is 20:4-10: 10‑20. Adding a certain proportion of crystal form stabilizer and basifying agent to the specific solvent of the present invention enables esomeprazole strontium or magnesium salt to obtain a pharmaceutical preparation with extremely high optical and crystal form purity. The preparation is safe and effective in clinical application, and solves the defects of sudden release risk and relatively large side effects after taking the drug that exist in similar products at present, and the product has higher quality stability.
Owner:JINAN KANGHE MEDICAL TECH
Enteric-coated tablet containing pantoprazole and preparation method thereof
ActiveCN104758267AFacilitated releaseImprove stabilityOrganic active ingredientsDigestive systemPharmaceutical formulationSodium salt
The invention discloses an enteric-coated tablet containing pantoprazole and a preparation method thereof. The enteric-coated tablet is prepared by wrapping a tablet core successively by an isolation coating and an enteric coating. The tablet core is prepared from following raw and auxiliary materials, by weight: 40 parts of the pantoprazole or a sodium salt thereof, 20-80 parts of sodium carbonate, 1-10 parts of sodium citrate, 0.1-1 part of vitamin E, 5-10 parts of xylitol, 20-40 parts of a filling agent and 1-5 parts of a lubricant. The enteric-coated tablet is quickly-released in intestinal juice, is higher than 90% in release rate after 30 min, is significantly improved in stability, and can be stored for 6 months at 40 DEG C under the humidity of 75% without different change in various quality indexes when compared with the quality indexes of a tablet just has been prepared. Meanwhile, the preparation method and the formula composition of the enteric-coated tablet are simple, so that industrial production of the tablet is easy to carry out.
Owner:LIAONING NIRVANA PHARMA
Faropenem sodium capsule
InactiveCN106890158AThe composition of the prescription is simpleSimple processAntibacterial agentsPharmaceutical non-active ingredientsChemistryCaplet Dosage Form
The invention provides a faropenem sodium capsule. The faropenem sodium capsule is characterized by being prepared from the following components in parts by weight on the basis of faropenem: 100 parts of faropenem sodium, 300-400 parts of filler, 8.0-80 parts of lubricant, 100-200 parts of dispersing agent and 10-50 parts of dry adhesive. The faropenem sodium capsule is prepared through a dry granulation technology, and the prepared faropenem sodium capsule solves the defect that the existing capsule is poor in stability.
Owner:HAINAN HONZ PHARMA
Arctiin compound entrapped local skin external preparation based on solid particle emulsification technology and preparation method and application of arctiin compound entrapped local skin external preparation
ActiveCN112823788AImprove permeabilityImprove retentionOrganic active ingredientsInorganic non-active ingredientsSkin permeabilityPharmaceutical drug
The embodiment of the invention relates to the field of preparations, in particular to an arctiin compound entrapped local skin external preparation based on a solid particle emulsification technology and a preparation method and application of the arctiin compound entrapped local skin external preparation. The arctiin compound entrapped external preparation provided by the embodiment of the invention comprises the following components in parts by weight: 1-15 parts of arctiin or arctigenin, 0.5-10 parts of a solid particle emulsifier, 10-40 parts of an oil phase, 5-30 parts of a solubilizer and 1-10 parts of a thickening agent, wherein the particle size of the solid particle emulsifier is nanometer or micrometer. According to the arctiin compound entrapped external preparation provided by the embodiment of the invention, the arctiin compound forms a stable and efficient system through the solid particle emulsifier, and can stably release medicine in the medication process. In addition, compared with a traditional emulsion, the external preparation also enhances the skin permeability and retention property of the arctiin compound, and shows better pharmacological activity and curative effect.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com