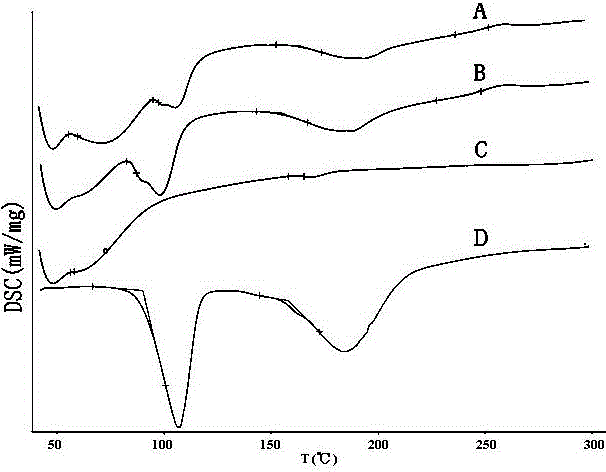
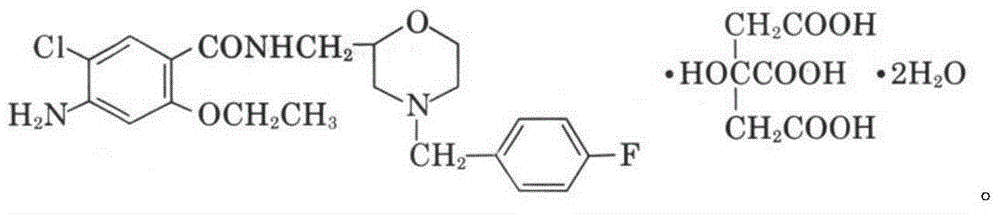
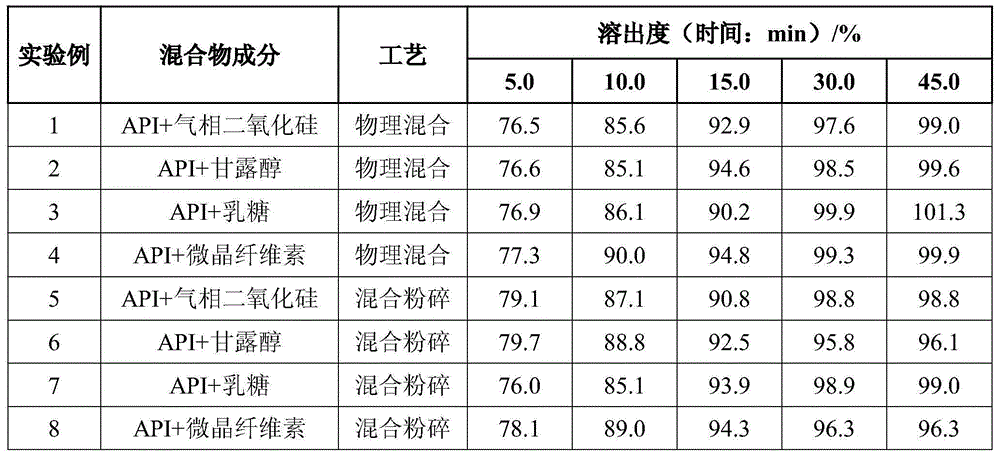
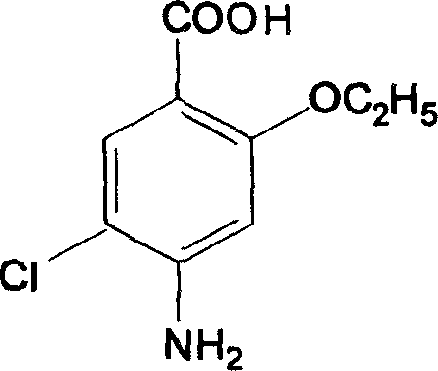
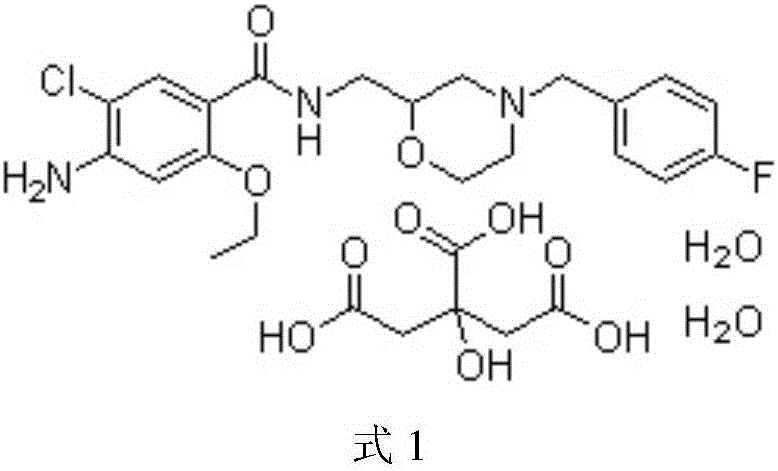
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
59 results about "Mosapride citrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
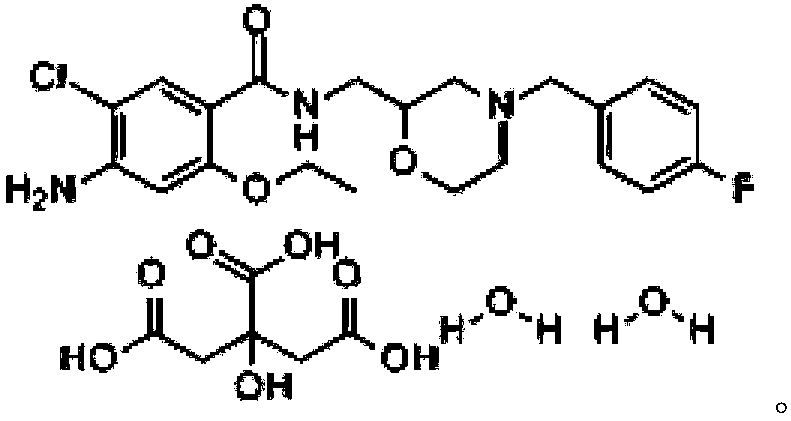
Mosapride Citrate is used for the treatment, control, prevention, & improvement of the following diseases, conditions and symptoms: Gastric acidity Digestive disorders Digestive disorder Mosapride Citrate may also be used for purposes not listed here.
Tablets of mosapride citrate and preparation method thereof
ActiveCN101816639ADissolution rate is fastReduce solubilityOrganic active ingredientsDigestive systemDissolutionIn vivo
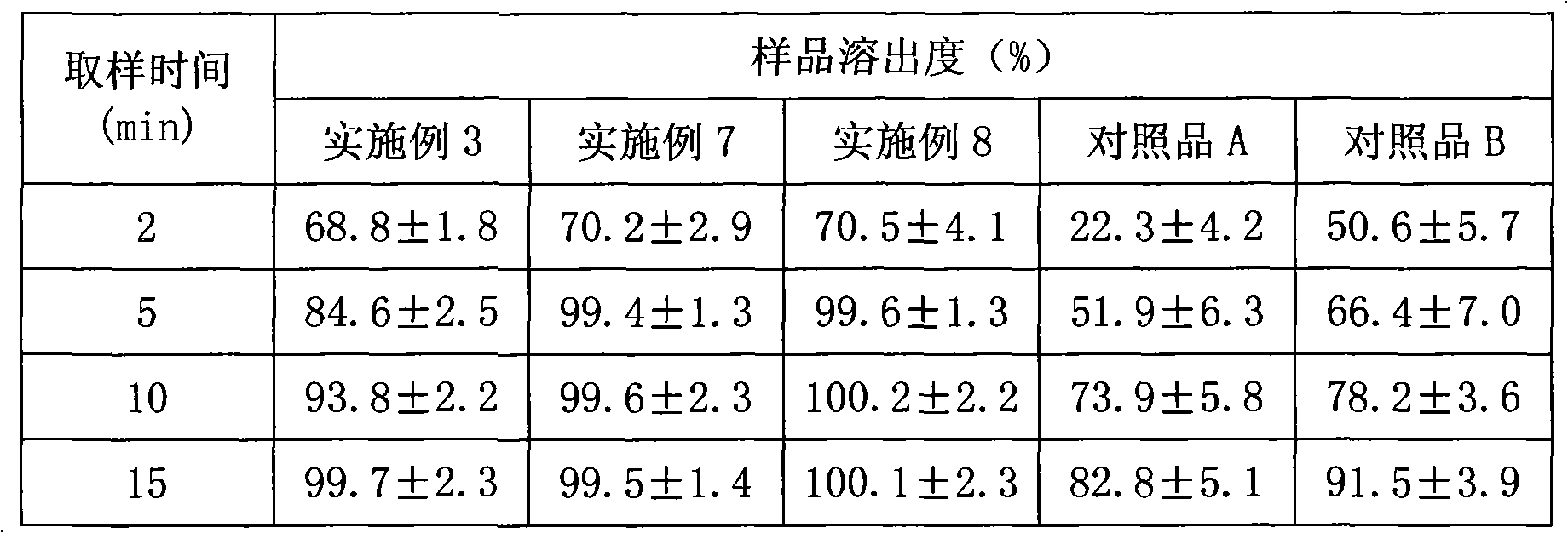
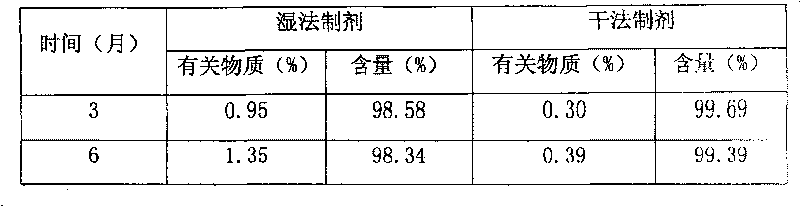
The invention belongs to the field of medicaments and in particular relates to tablets containing mosapride citrate and a preparation method thereof. Because the mosapride citrate is almost insoluble, defects of slow dissolution and low in-vitro and in-vivo bioavailability are present in the mosapride citrate tablets prepared by the common method. In order to improve the dissolution and bioavailability of the mosapride citrate, the invention provides dispersible tablets containing the mosapride citrate and a preparation method thereof. The prepared mosapride citrate dispersible tablets have high dissolution speed and low dissolution difference. The accumulated dissolution in five minutes is close to 100 percent, and the dispersible tablets are almost dissolved completely.
Owner:LUNAN PHARMA GROUP CORPORATION
Pharmaceutical combination containing Mosapride citrate and method of preparing the same
ActiveCN101273973ASolve technical problemsQuality improvementOrganic active ingredientsDigestive systemMedicineAdhesive
The invention relates to a pharmaceutical composition containing mosapride citrate which is applicable to the usage of direct powder compression for preparation and a preparation method thereof. The pharmaceutical composition further contains a disintegrant, a thinner, a lubricant, a glidant and an adhesive in addition to the active component of the mosapride citrate. The pharmaceutical composition is applicable to the preparation of dispersible tablets which can be fully disintegrated in 2 minutes in water under the temperature of 19 DEG C to 21 DEG C and pass through a No. 2 screen, the hardness is 8 to 11Kg, and the dissolution rate is in line with the relevant provisions, thus solving the problems of higher relevant substances, instable quality and difficult packaging of the mosapride citrate dispersible tablets prepared by wet granulation.
Owner:CHENGDU KANGHONG PHARMA GRP
Mosapride citrate sustained-release tablet
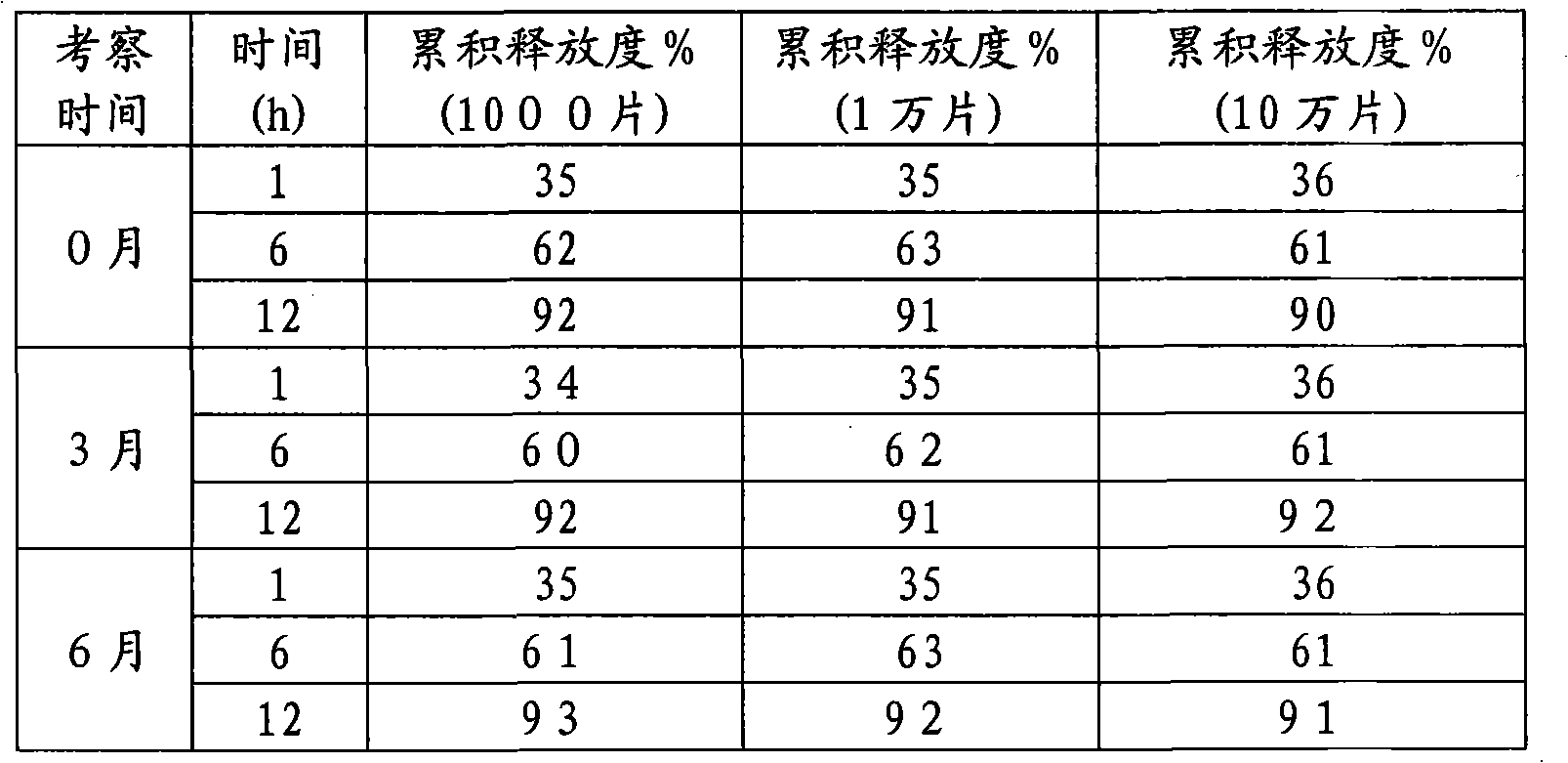
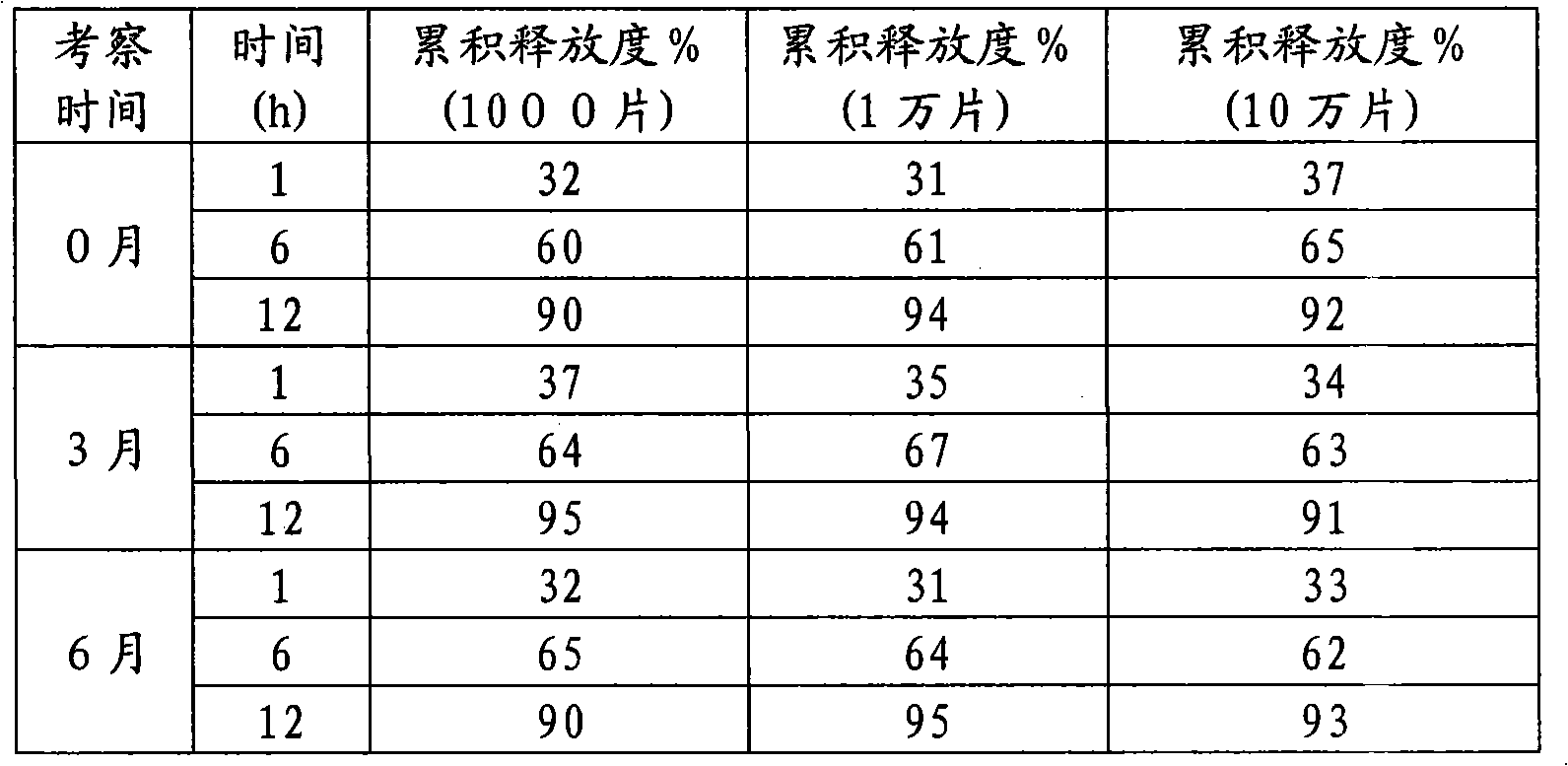
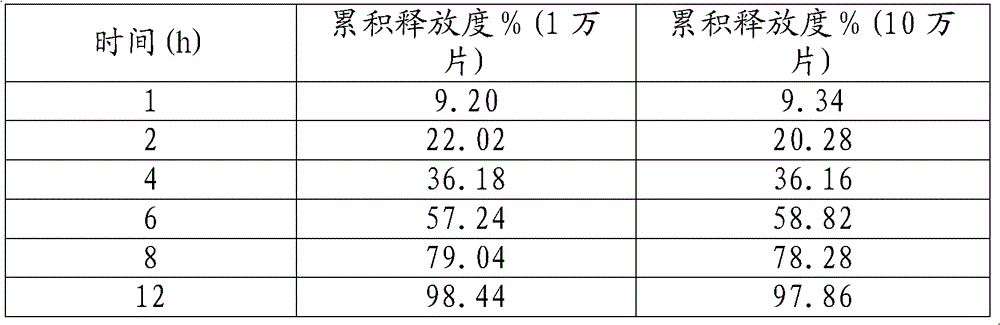
The invention discloses a mosapride citrate sustained-release tablet. The mosapride citrate sustained-release tablet comprises mosapride citrate, slow-release materials, a diluent, an adhesive, a lubricant and a coating material. A release degree of the mosapride citrate sustained-release tablet has good repeatability, regardless of being detected in an experiment or in mass production. After being subjected to stability monitoring for 6 months, the mosapride citrate sustained-release tablet still has an almost changeless release degree.
Owner:重庆健能医药开发有限公司
Pharmaceutical combination containing Mosapride citrate
ActiveCN101273973BSolve the problem of highFix stability issuesOrganic active ingredientsDigestive systemActive componentAdhesive
The invention relates to a pharmaceutical composition containing mosapride citrate which is applicable to the usage of direct powder compression for preparation and a preparation method thereof. The pharmaceutical composition further contains a disintegrant, a thinner, a lubricant, a glidant and an adhesive in addition to the active component of the mosapride citrate. The pharmaceutical composition is applicable to the preparation of dispersible tablets which can be fully disintegrated in 2 minutes in water under the temperature of 19 DEG C to 21 DEG C and pass through a No. 2 screen, the hardness is 8 to 11Kg, and the dissolution rate is in line with the relevant provisions, thus solving the problems of higher relevant substances, instable quality and difficult packaging of the mosapridecitrate dispersible tablets prepared by wet granulation.
Owner:CHENGDU KANGHONG PHARMA GRP
Synthesis of Important intermediate for mosapride citrate
InactiveCN1526700AWide variety of sourcesRealize localizationOrganic compound preparationCarboxylic acid amides preparationAcetic anhydrideMorpholine
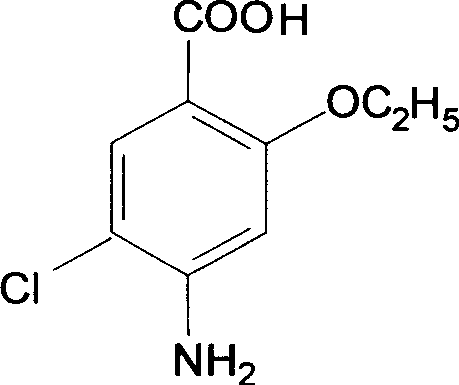
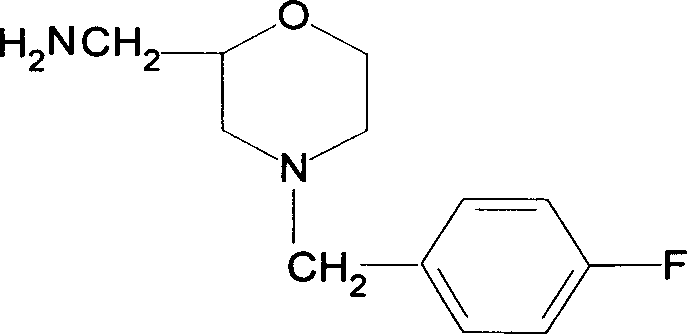
The present invention relates to the preparation process of important intermediates for Mosapride citrate, 2-oxethyl-4-actamino-5-chlorobenzoic acid and 4-(4-fluorobenzyl-2- aminomethyl morpholine. The intermediate 2-oxethyl-4-actamino-5-chlorobenzoic acid is prepared with amino salicylic acid as initial material and through acidification with hydrochloric acid, esterification with methol, acetylation with acetic anhydride, ethylation, NCS chlorination and alkali hydrolysis. The intermediate 4-(4-fluorobenzyl-2- aminomethyl morpholine is prepared with p-fluorobenzaldehyde and phthalimide as initial material and through dropping at 70-90 deg.c, maintaining at 125-145 deg.c, and post-treatment in ice bath in 2-10 deg.c while adding acetic anhydride through stirring for 10-20hr. The present invention can raise the yield of Mosapride citrate and lower its production cost.
Owner:LUNAN BETTER PHARMA
Tablets of mosapride citrate and preparation method thereof
ActiveCN101816639BDissolution rate is fastReduce solubilityOrganic active ingredientsDigestive systemMosapride citrateMedicinal chemistry
The invention belongs to the field of medicaments and in particular relates to tablets containing mosapride citrate and a preparation method thereof. Because the mosapride citrate is almost insoluble, defects of slow dissolution and low in-vitro and in-vivo bioavailability are present in the mosapride citrate tablets prepared by the common method. In order to improve the dissolution and bioavailability of the mosapride citrate, the invention provides dispersible tablets containing the mosapride citrate and a preparation method thereof. The prepared mosapride citrate dispersible tablets have high dissolution speed and low dissolution difference. The accumulated dissolution in five minutes is close to 100 percent, and the dispersible tablets are almost dissolved completely.
Owner:LUNAN PHARMA GROUP CORPORATION
Mosapride citrate tablet and preparation method thereof
The invention discloses a mosapride citrate tablet. The preparation method comprises the following steps: dissolving citric acid in ethanol, adding raw materials of mosapride citrate, stirring until the raw materials are dissolved, and drying the solution to obtain a mixture; mixing the mixture with pharmaceutically acceptable accessories and tabletting. Compared with the prior art, the tablet disclosed by the invention can be rapidly dissolved in water; and the preparation process is simple.
Owner:LUNAN PHARMA GROUP CORPORATION
Mosapride citrate dehydrate sustained release tablet
ActiveCN103356498AGuaranteed reproducibilityGuaranteed uniformityOrganic active ingredientsDigestive systemSustained Release TabletMedicine
The invention discloses a mosapride citrate dehydrate sustained release tablet. The sustained release tablet comprises the following components according to a unit dose package: 15mg of citric acid mosapride, 30-50mg of sustained-release materials, 30-70mg of a stabilizer, a bonding agent, a lubricant and a coating material. The sustained release tablet has a 12-hour sustained release effect and good chemical stability.
Owner:四川健能制药有限公司
Pharmaceutical composition containing mosapride citrate
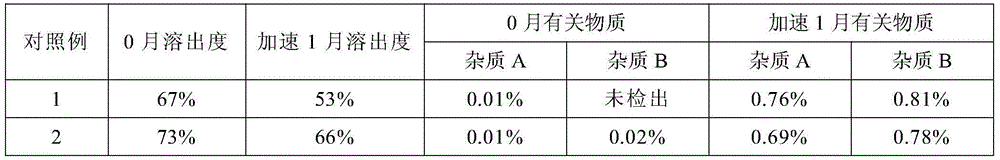
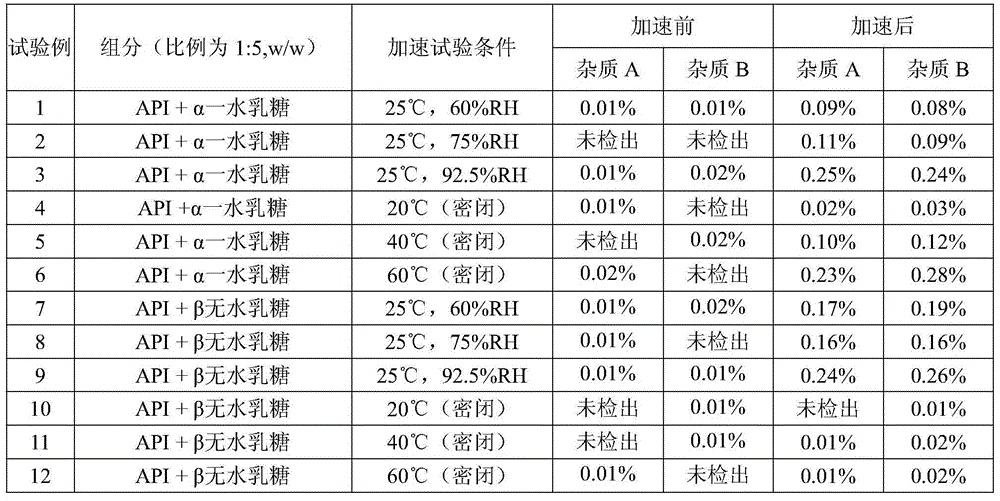
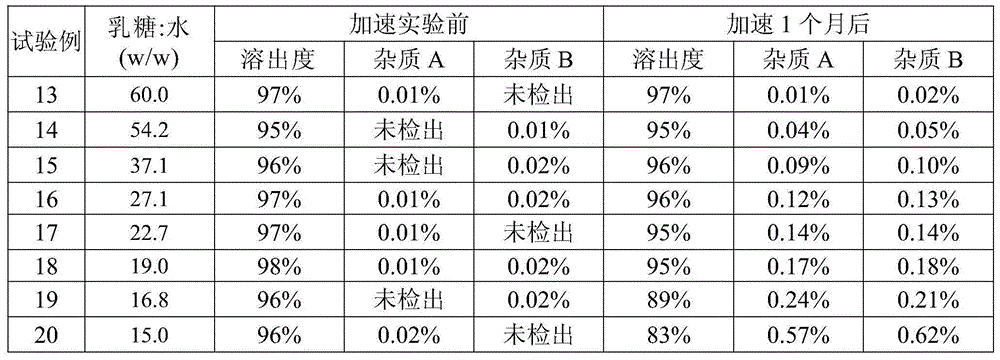
The invention relates to a pharmaceutical composition containing mosapride citrate. The pharmaceutical composition contains lactose and other pharmaceutically acceptable accessory materials. Through strict control of a mass ratio of lactose to water in the pharmaceutical composition, mosapride citrate long-term storage stability is greatly improved and related substance generation and increasing risk is reduced. The pharmaceutical composition provides guarantee for clinical drug safety and effectiveness.
Owner:CHENGDU KANGHONG PHARMA GRP
Mosapride citrate co-grinded product, preparation method thereof and medicine composition containing mosapride citrate co-grinded product
ActiveCN104546686AImprove solubilityImprove dissolution rateOrganic active ingredientsDigestive systemMedicineLow-substituted hydroxypropylcellulose
The invention discloses a mosapride citrate co-grinded product, a preparation method thereof and a medicine composition containing the mosapride citrate co-grinded product. The mosapride citrate co-grinded product is prepared by co-grinding mosapride citrate with a hydrophilic macromolecular auxiliary material in a mass ratio of 10-55%: 90-45%, wherein the hydrophilic macromolecular auxiliary material is selected from one or more of beta-cyclodextrin, low-substituted hydroxypropyl cellulose, hydroxypropyl methylcellulose, povidone and polyethylene glycol 6000. The medicine composition prepared by virtue of the mosapride citrate co-grinded product is capable of remarkably improving the dissolution rate of mosapride citrate, simple in preparation process, and suitable for industrialized production.
Owner:南京百思福医药科技有限公司
Rapidly-dissolving mosapride citrate composition
ActiveCN105769872AReduce manufacturing costImprove securityOrganic active ingredientsDigestive systemAdditive ingredientAdhesive
The invention provides a rapidly-dissolving mosapride citrate composition, which comprises mosapride citrate and fumed silica. Mosapride citrate and fumed silica are mixed and crushed in a crushing machine, wherein mass ratio of mosapride citrate to fumed silica is 1:0.2-1.2; and particle size D50 of the mosapride citrate is less than or equal to 3.0 microns. The composition also contains one or more ingredients selected from a filler, a disintegrating agent, an adhesive, a lubricant, a flow aid and a flavouring agent. By the use of the mosapride citrate composition, dissolution rate of mosapride citrate in a buffer solution (a simulated intestinal fluid) with pH 6.8 is greatly raised, and medication effectiveness is raised for achlorhydria patients. The preparation technology is simple and is easy to operate. Any organic reagents are not used, and hot and humid process is not required. Instability of mosapride citrate in a hot and humid environment is avoided. The composition of the invention is suitable for industrial production.
Owner:CHENGDU KANGHONG PHARMA GRP
Mosapride citrate tablet and preparation method thereof
ActiveCN104622826AAdvantages and Significant AdvancementsPromote dissolutionOrganic active ingredientsDigestive systemMedicineTableting
The invention discloses a mosapride citrate tablet and a preparation method thereof. The tablet is obtained by virtue of wet granulation and tableting; a binder adopted for wet granulation is the aqueous solution of mosapride citrate and povidone K15. Compared with the prior art, the mosapride citrate tablet is quick in drug dissolution, stable in quality, simple in preparation process without complex preparation equipment, and prone to industrial mass production.
Owner:SHANGHAI PUKANG PHARMA
Mosapride citrate granules and preparation method thereof
ActiveCN104940148ARapid dissolutionSuitable for long term storageOrganic active ingredientsPharmaceutical non-active ingredientsMedicinePolyethylene glycol
The invention discloses mosapride citrate granules and a preparation method thereof, and relates to a granule agent containing polyethylene glycol, hydroxypropyl cellulose and mosapride citrate. The granule agent uses hydroxypropyl cellulose as carriers, uses polyethylene glycol as solubilizer, and then is combined with pharmacy acceptable auxiliary materials, dissolving experiments show that mosapride citrate is basically all dissolved at the moment of 5 min, acceleration experiments show that the stability of mosapride citrate is high, and the preparation technology is simple and suitable for industrial mass production.
Owner:LUNAN PHARMA GROUP CORPORATION
Synthesis of Important intermediate for mosapride citrate
InactiveCN1226295CHigh synthetic yieldImprove composite qualityOrganic compound preparationCarboxylic acid amides preparationAcetic anhydrideN-Chlorosuccinimide
The present invention relates to the preparation process of important intermediates for Mosapride citrate, 2-oxethyl-4-actamino-5-chlorobenzoic acid and 4-(4-fluorobenzyl-2- aminomethyl morpholine. The intermediate 2-oxethyl-4-actamino-5-chlorobenzoic acid is prepared with amino salicylic acid as initial material and through acidification with hydrochloric acid, esterification with methol, acetylation with acetic anhydride, ethylation, NCS chlorination and alkali hydrolysis. The intermediate 4-(4-fluorobenzyl-2- aminomethyl morpholine is prepared with p-fluorobenzaldehyde and phthalimide as initial material and through dropping at 70-90 deg.c, maintaining at 125-145 deg.c, and post-treatment in ice bath in 2-10 deg.c while adding acetic anhydride through stirring for 10-20hr. The present invention can raise the yield of Mosapride citrate and lower its production cost.
Owner:LUNAN BETTER PHARMA
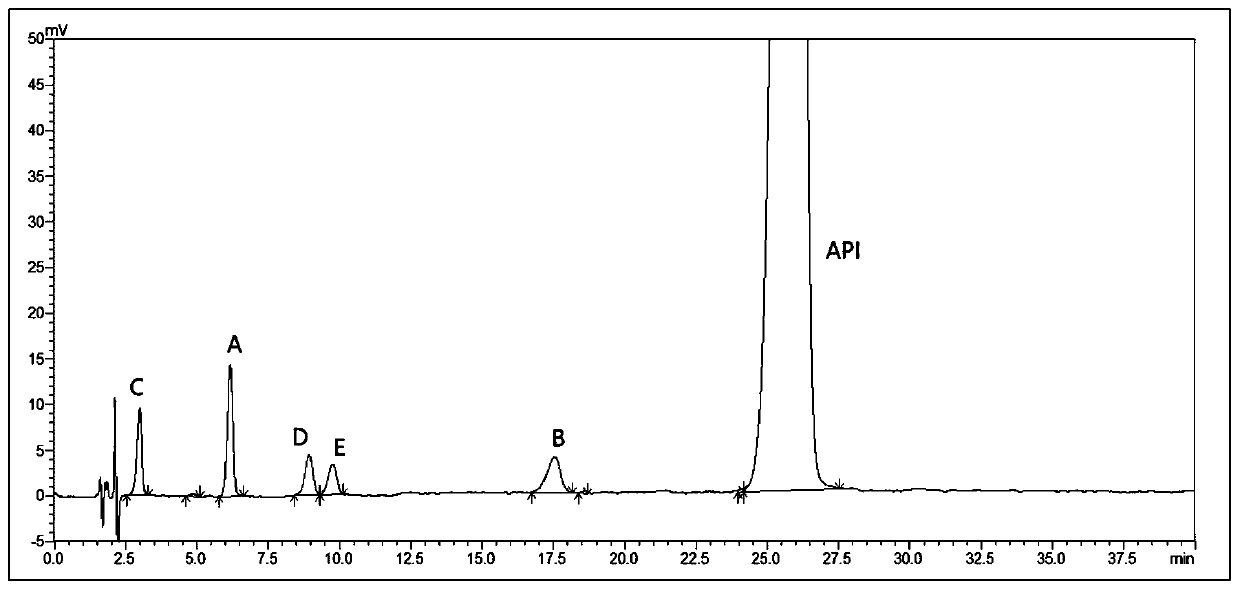
Mosapride citrate related substance detection method
ActiveCN105301118AEasy to separateAccurate detectionComponent separationGradient elutionMosapride citrate
The invention provides a mosapride citrate related substance detection method which comprises the step of detecting related substances in the pharmaceutical composition (mosapride citrate) through a high performance liquid chromatography and a gradient elution method. The detection method provided invention has the advantages that the number of impurities detected by the detection method is maximum, the degree of separation is relatively good, in addition, the response value is high and the sensitivity is good.
Owner:CHENGDU KANGHONG PHARMA GRP
Mosapride citrate film-coated tablet and preparation method thereof
ActiveCN106214657AImprove stabilityGood lookingOrganic active ingredientsDigestive systemDissolutionBULK ACTIVE INGREDIENT
The invention provides a pharmaceutical composition comprising active ingredient mosapride citrate and a preparation method thereof. Particularly, the pharmaceutical composition of mosapride citrate prepared herein meets the coating requirement, the dissolution of pharmaceutical composition of mosapride citrate in different media is improved, and the stability of the composition in the illumination stage is improved.
Owner:JIANGSU HANSOH PHARMA CO LTD
Film-coated tablet of mosapride citrate and preparation method thereof
ActiveCN106214657BGood lookingOrganic active ingredientsDigestive systemDissolutionBULK ACTIVE INGREDIENT
The invention provides a pharmaceutical composition comprising active ingredient mosapride citrate and a preparation method thereof. Particularly, the pharmaceutical composition of mosapride citrate prepared herein meets the coating requirement, the dissolution of pharmaceutical composition of mosapride citrate in different media is improved, and the stability of the composition in the illumination stage is improved.
Owner:JIANGSU HANSOH PHARMA CO LTD
Mosapride Citrate Sustained Release Tablets
ActiveCN103356498BGuaranteed reproducibilityGuaranteed uniformityOrganic active ingredientsDigestive systemSustained Release TabletMedicine
The invention discloses a mosapride citrate dehydrate sustained release tablet. The sustained release tablet comprises the following components according to a unit dose package: 15mg of citric acid mosapride, 30-50mg of sustained-release materials, 30-70mg of a stabilizer, a bonding agent, a lubricant and a coating material. The sustained release tablet has a 12-hour sustained release effect and good chemical stability.
Owner:四川健能制药有限公司
Mosapride citrate pharmaceutical composition
ActiveCN110354093ARelease stabilityImprove stabilityOrganic active ingredientsDigestive systemSide effectDiluent
The invention discloses a mosapride citrate pharmaceutical composition. The composition comprises mosapride citrate, a slow-release agent, a diluent, a flow aid, a lubricant and the like. The composition is gentle in release rate, free of initial burst release effect, high in stability, capable of improving the medication compliance of a patient, high in medication safety and small in toxic and side effect.
Owner:CHANGZHOU HANSOH PHARM CO LTD
Method for preparing mosapride citrate intermediate
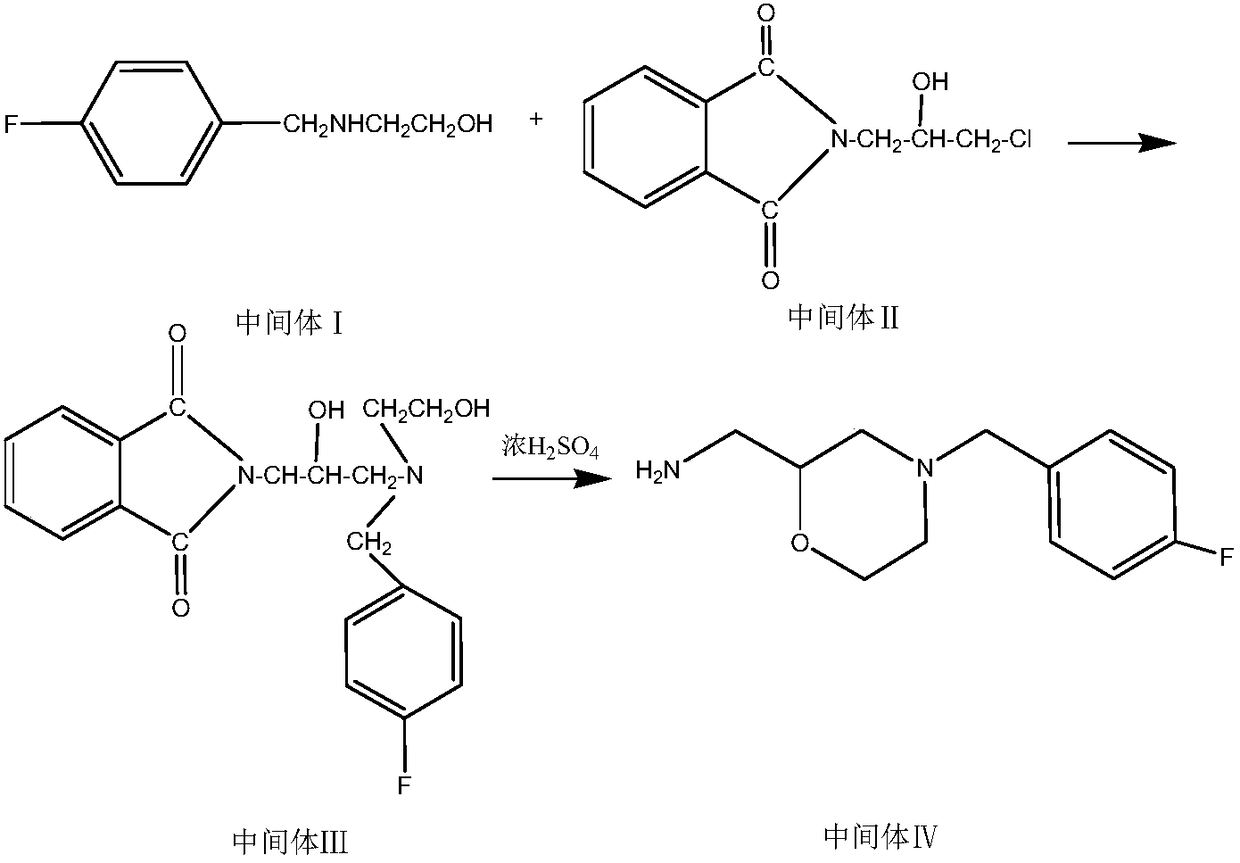
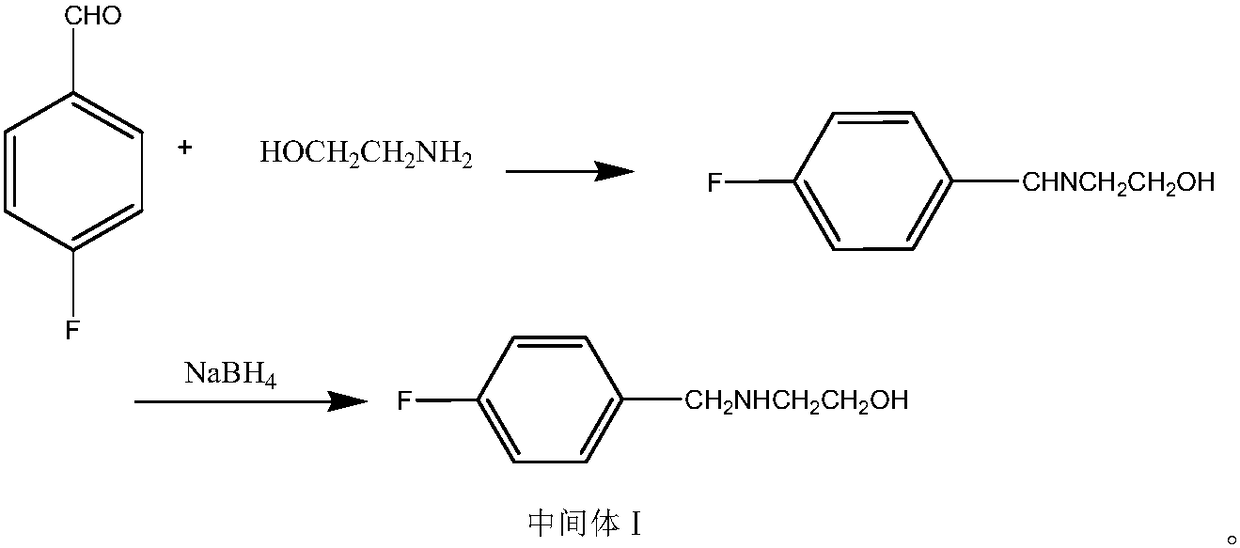
The invention belongs to the technical field of medicines and particularly relates to a method for preparing a mosapride citrate intermediate IV 4-(4-fluorophenyl)-2-aminomethyl morpholine. The methodcomprises the following steps: phthalimide potassium salt and dichloro-2-propanol react to produce an intermediate II N-(2-hydroxy-3-chloropropyl) phthalimide, then the produced intermediate II and an intermediate I 2-(4-fluorphenylamine)ethyl alcohol are condensed to prepare an intermediate III N-3-[4-fluorophenyl-2-(hydroxy-ethylamine)-2-hydroxypropyl]phthalic diamide, and the intermediate IIIis subjected to cyclization and hydrolysis to obtain the intermediate IV 4-(4-fluorophenyl)-2-aminomethyl morpholine. The method is short in route, lower in production cost and suitable for industrialproduction.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method of mosapride citrate stomach buoyant sustained-release pellet
ActiveCN109010319AControl releaseRapidly hydrates and swellsOrganic active ingredientsDigestive systemSustained release pelletsControlled release
The invention discloses a preparation method of a mosapride citrate stomach buoyant sustained-release pellet. The mosapride citrate stomach buoyant sustained-release pellet is formed by a drug-loadedpellet core, and a gas-generating layer and a retardation layer which wrap the pellet core, the inner layer of the pellet is a light-weight pellet core, the outer layer of the pellet is wrapped with acontrolled-release polymer material, and the middle layer of the pellet is the gas-generating layer produced from NaHCO3 and HPMC used as an adhesive. When the pellet meets a release medium water, the pellet can be quickly hydrated and expand, the pellet and CO2 gas produced by NaHCO3 in the gas-generating layer are prevented from overflowing by the retardation layer, so the pellet can float fora long time, and the release of the drug from the core is controlled; and the mosapride citrate stomach buoyant sustained-release pellet has the advantages of good roundness, good buoyancy in vitro, realization of a 8 h buoying rate reaching 80% or above, and good sustained-release characteristic.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
Capsule containing mosapride citrate and preparation method thereof
ActiveCN108324697ARapid dissolutionHigh dissolution rateOrganic active ingredientsDigestive systemDissolutionMedical prescription
The invention relates to a capsule containing mosapride citrate and a preparation method thereof. The capsule includes drug granules and a capsule casing. The drug granules contain the mosapride citrate and / or a mosapride citrate dihydrate, a diluent, a binder, a disintegrating agent and a lubricant. The mosapride citrate in the capsule can be quickly dissolved out at high dissolution rate, so that bioavailability is increased. The formula and the preparation method are simple, have high controllability and low production cost, and are easy to achieve in industrial production.
Owner:科贝源(北京)生物医药科技有限公司
Citric acid Mosabily oral liquid
An orally taken solution of mosapride citrate is prepared from mosapride citrate, glycerin, alcohol, propanediol, polyethanediol-300, polyethanediol-400, antisetic and flavouring. It has fast absorption rate and high stability.
Owner:LUNAN PHARMA GROUP CORPORATION
Mosapride citrate tablet and preparation method thereof
ActiveCN107744509AAdvantages and Significant AdvancementsPromote dissolutionOrganic active ingredientsDigestive systemDissolutionLactose
The invention relates to a mosapride citrate tablet and a preparation method thereof, and belongs to the technical field of medical preparations which are applied to medical use and contain organic active ingredients. With mosapride citrate as a raw material and lactose, starch and hydroxypropyl methylcellulose as adjuvant materials, and assisted by aids, the mosapride citrate tablet is prepared by conducting wet-process granulation and tabletting. The mosapride citrate tablet provided by the invention, when applied to the preparation of gastric motor drugs, has the characteristics of being rapid in dissolution, good in stability, simple in preparation process, easy for industrial mass production and the like.
Owner:ZHEJIANG ANGLIKANG PHARMA
Method for detecting mosapride citrate and five key impurities in preparation of mosapride citrate
The invention discloses a method for detecting mosapride citrate and five key impurities in a preparation of the mosapride citrate, and belongs to the technical field of medicine quality detection methods, and particularly relates to the method for detecting mosapride citrate and five key impurities in the preparation of the mosapride citrate by adopting high performance liquid chromatography. According to the detection method, the test solution is prepared, the isocratic elution is carried out by adopting the high performance liquid chromatography, and the related substances in a drug and thepreparation of the drug are detected. The detection method is good in separation degree, high in sensitivity on the related substances, stable in baseline and short in analysis time, and is simple and convenient to operate.
Owner:南京百思福医药科技有限公司
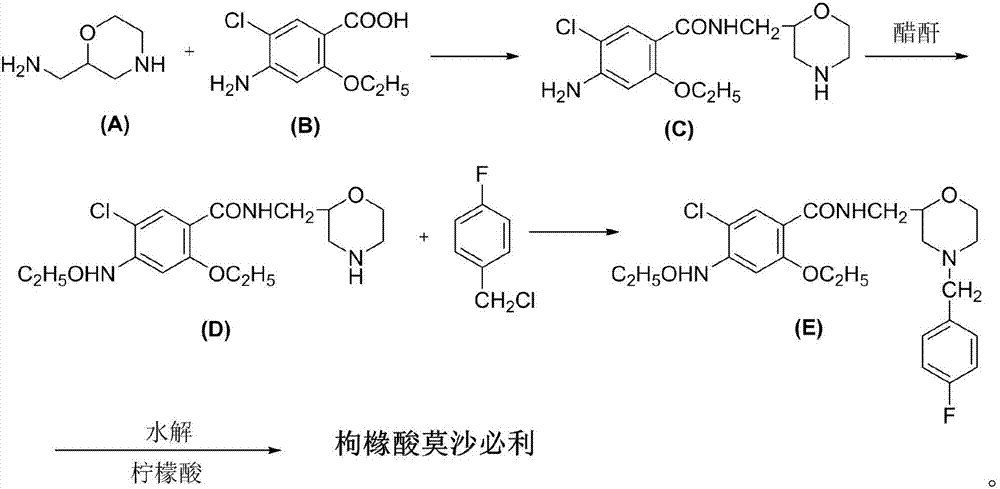
Green environment-friendly synthetic method for mosapride citrate
InactiveCN107513044AReduce productionIncrease profitOrganic chemistryBulk chemical productionBenzoic acidAcetic anhydride
The invention discloses a green environment-friendly synthetic method for mosapride citrate and relates to the technical field of organic synthesis. The method comprises the following steps: by taking 2-aminomethyl morpholine as a raw material, performing condensation reaction on the 2-aminomethyl morpholine and 5-chlorine-4-amino-2-ethyoxyl benzoic acid, thereby acquiring a midbody (C); utilizing acetic anhydride to protect an amino group on benzene ring of the midbody (C), thereby acquiring a midbody (D); performing alkylation reaction on the midbody (D) and fluorobenzyl chloride in the presence of inorganic alkali, thereby acquiring a midbody (E); hydrolyzing amino protection group of the midbody (E) in a citric acid aqueous solution and salifying with citric acid, thereby acquiring a crude product of mosapride citrate; and refining the crude product of mosapride citrate with ethyl alcohol, thereby acquiring a pure product of mosapride citrate. According to the invention, the low-cost easily acquired 2-aminomethyl morpholine is used for directly introducing morpholine group, so that the output of three wastes is obviously reduced, the use rate of the equipment is increased and the purpose of green environment-friendly production is achieved.
Owner:安徽修一制药有限公司
Medicine composition of mosapride citrate and preparation method of same
ActiveCN106943369AImprove thermal stabilityHides bitterness wellOrganic active ingredientsDigestive systemPlasticizerBitter taste
The invention provides a medicine composition containing mosapride citrate and a preparation method of same. In particular, the medicine composition contains the mosapride citrate and a plasticizer. The medicine composition has high preparation stability, can mask the bitter taste of the mosapride citrate well and also improves dissolution behavior of the mosapride citrate in different mediums.
Owner:JIANGSU HANSOH PHARMA CO LTD
Mosapride citrate preparation and preparation method thereof
ActiveCN107684548AImprove solubilityImprove stabilityOrganic active ingredientsDigestive systemSoft materialsMedical prescription
The invention provides a mosapride citrate preparation and a preparation method thereof. The method comprises the following steps: firstly, forming an inclusion compound by hydroxypropyl-beta-cyclodextrin and mosapride citrate; then uniformly mixing the inclusion compound and pharmaceutical excipients; adding a proper amount of a binding agent to prepare a soft material; after granulating and drying, adding a lubricant; pelleting and carrying out tabletting. Mosapride citrate dispersed tablets prepared by the preparation method have the advantages of rapid disintegration speed and good stability, good mouthfeel, simple prescription, good flowability of mixed powder in the prescription, no sticking in a tabletting process and the like and the mosapride citrate preparation is widely appliedto the field of preparations of the mosapride citrate dispersed tablets. The preparation method has the advantages that a technological flow is effectively shortened, the production period is shortened and the exposure time of medicines in the air is reduced so that the stability of the medicines is ensured, and furthermore, the quality of products is improved.
Owner:LUNAN BETTER PHARMA
Mosapride citrate tablet and preparation method thereof
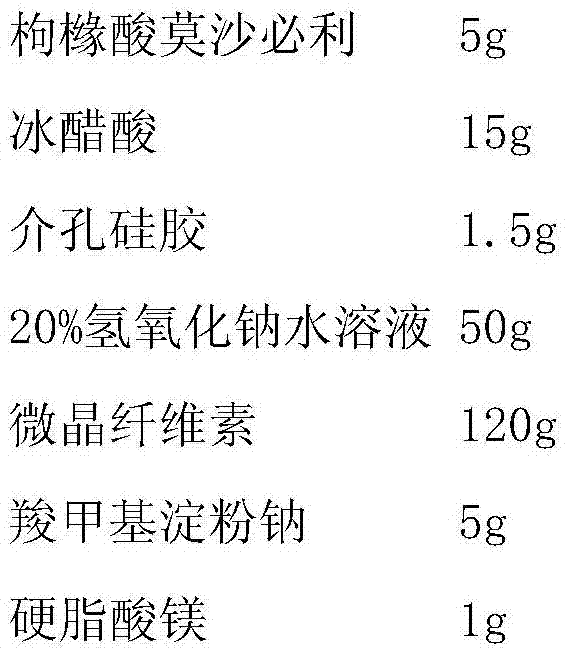
ActiveCN106913879APromote dissolutionSimple preparation processOrganic active ingredientsDigestive systemAcetic acidAqueous sodium hydroxide
The invention discloses a mosapride citrate tablet and a preparation method thereof. The preparation method comprises the following steps: dissolving mosapride citrate glacial acetic acid; adding mesoporous silica and carrying out uniform mixing under stirring; then adding an aqueous sodium hydroxide solution into a solution obtained in the previous step under stirring; allowing a compound of mosapride citrate and mesoporous silica to be precipitated and carrying out filtering and drying; then mixing the dried compound with a filler and a disintegrating agent and carrying out granulation and drying; and adding a lubricant into dry particles and then carrying out tabletting. Compared with the prior art, the mosapride citrate tablet prepared in the invention can be rapidly dissolved out in water, and the preparation method is simple in process and suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
Oral quick-release membrane containing mosapride citrate solid dispersion
InactiveCN109700786AImprove complianceFacilitated releaseOrganic active ingredientsDigestive systemPatient complianceSODIUM SACCHARIN
The invention discloses an oral quick-release membrane containing mosapride citrate solid dispersion. The membrane is prepared from the following raw material medicines and auxiliary materials in parts by weight: 3.0-8.0 parts of mosapride citrate, 10.0-20.0 parts of hydroxypropyl cellulose, 20.0-44.0 parts of hydroxypropyl methyl cellulose, 10.0-15.0 parts of methyl cellulose, 0.5-1.0 parts of carbomer, 9.0-28.0 parts of a plasticizer, and 1.0-4.0 parts of sodium saccharin. The oral quick-release membrane containing mosapride citrate solid dispersion of the invention can be conveniently applied to patients with dysphagia, the medicine release is rapid, the bioavailability and patient compliance are improved, the medicines exist in a preparation in an amorphous form, the shelf life of themedicines can be prolonged, and the effect of masking the bad taste of the medicines is realized.
Owner:重庆健能医药开发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com