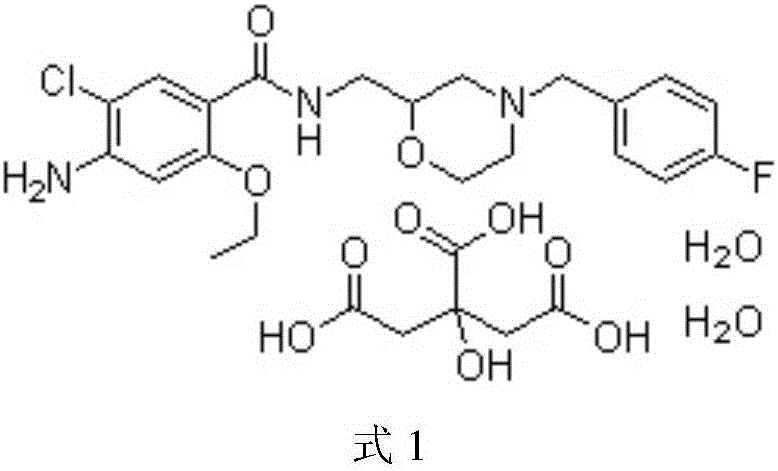
Mosapride citrate film-coated tablet and preparation method thereof
A mosapride citrate and film coating technology is applied in the field of pharmaceutical compositions containing mosapride citrate and its preparation, which can solve the problems of reducing productivity and affecting the appearance of film-coated tablets, etc., to achieve Excellent stability and superior storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1 (sample 4)
[0096] [Table 7] Chip composition
[0097]
[0098] [Table 8] Composition of film coating aqueous solution
[0099]
[0100] The plain tablet (core tablet) containing mosapride citrate was prepared according to the composition ratio shown in the above table [7].
[0101] 40g of purified water was added to the mixture containing mosapride citrate (5.29g), lactose (61.91g), starch (32.4g) and low-substituted hydroxypropyl cellulose (9.5g) for wet granulation, After drying, pass through a 30-mesh sieve to sieve and blend low-substituted hydroxypropylcellulose (9g), magnesium stearate (1.3g) and silicon dioxide (0.6g). Then the obtained granules are pressed into a plain tablet with a hardness of 3-10 kg using a punching film with a diameter of 6.5 mm.
[0102] The plain tablets (core tablets) obtained above were sprayed with the film coating solution having the ingredients shown in Table [8] to obtain film-coated tablets (about 124 g per t...
Embodiment 2
[0103] Embodiment 2 (sample 5)
[0104] [Table 9] Chip composition
[0105]
[0106]
[0107] [Table 10] Composition of film coating aqueous solution
[0108]
[0109] The plain tablet (core tablet) containing mosapride citrate was prepared according to the composition ratio shown in the above table [9].
[0110] Add 40g of purified water to the mixture containing mosapride citrate (5.29g), lactose starch (92.81g) and low-substituted hydroxypropyl cellulose (14g) for wet granulation, and pass through a 30-mesh sieve after drying The granules were sized and blended with low-substituted hydroxypropylcellulose (6g) and magnesium stearate (1.9g). Then the obtained granules are pressed into a plain tablet with a hardness of 3-10 kg using a punching film with a diameter of 6.5 mm.
[0111] The plain tablets (core tablets) obtained above were sprayed with the film coating solution having the ingredients shown in Table [10] to obtain film-coated tablets (about 124 g per ta...
Embodiment 3
[0112] Embodiment 3 (sample 6)
[0113] [Table 11] Chip composition
[0114]
[0115] [Table 12] Composition of film coating aqueous solution
[0116]
[0117]
[0118] The plain tablet (core tablet) containing mosapride citrate was prepared according to the composition ratio shown in the above table [11].
[0119] 40g of purified water was added to the mixture containing mosapride citrate (5.29g), lactose (61.91g), starch (32.4g) and low-substituted hydroxypropyl cellulose (9.5g) for wet granulation, After drying, pass through a 30-mesh sieve to sieve and blend low-substituted hydroxypropylcellulose (9g), magnesium stearate (1.3g) and silicon dioxide (0.6g). Then the obtained granules are pressed into a plain tablet with a hardness of 3-10 kg using a punching film with a diameter of 6.5 mm.
[0120] The plain tablets (core tablets) obtained above were sprayed with the film coating solution having the ingredients shown in Table [12] to obtain film-coated tablets (a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com