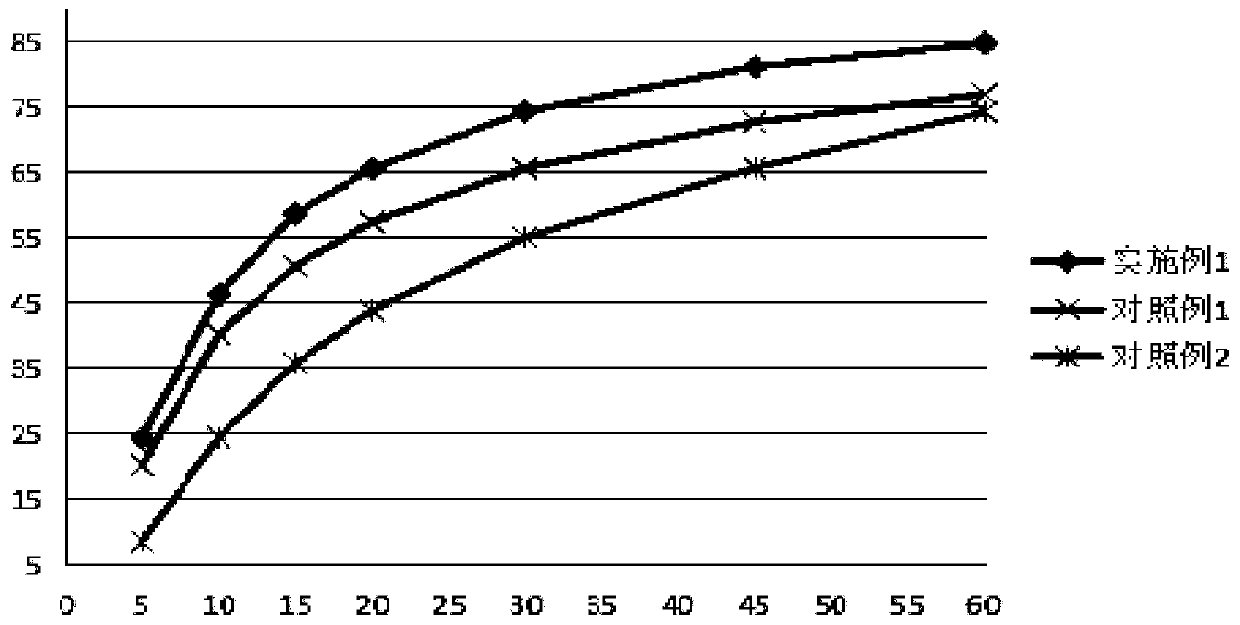
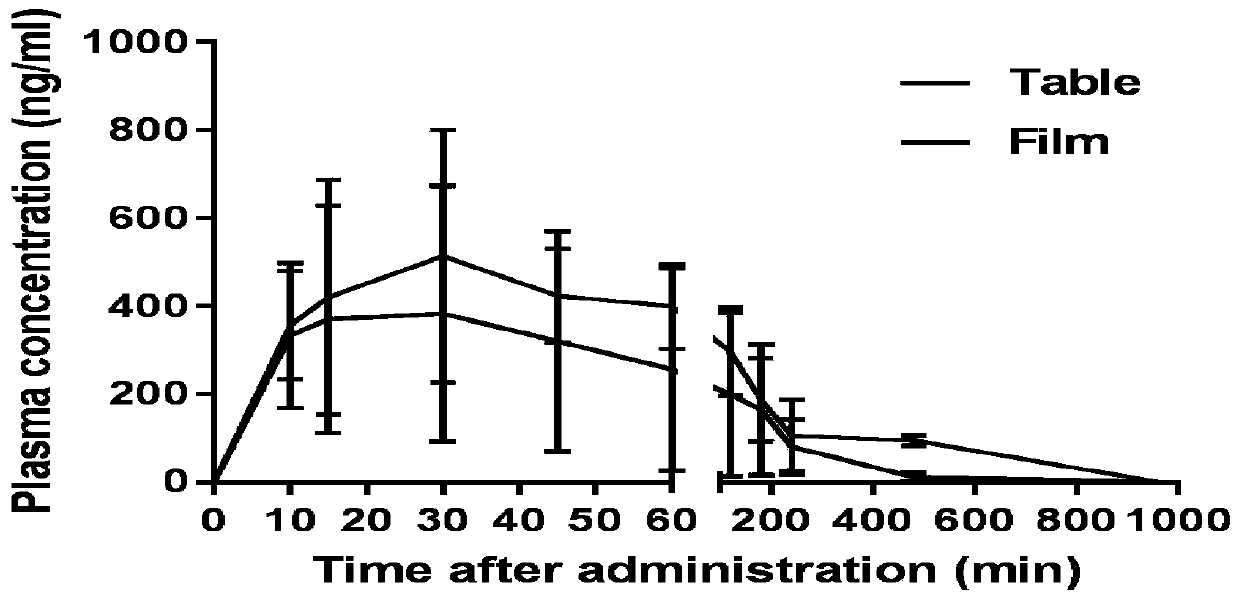
Oral quick-release membrane containing mosapride citrate solid dispersion
A technology of mosapride citrate and solid dispersion, applied in the field of preparation, can solve the problems of low bioavailability, poor patient compliance, frequent administration, etc., achieve rapid drug release, improve patient compliance, and mask drugs Effects of bad taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of oral immediate-release film containing mosapride citrate solid dispersion Prepare 100 tablets, each containing 5 mg of mosapride citrate, the amount of raw materials and auxiliary materials is: mosapride citrate Puridine 0.5g, hydroxypropylcellulose 1.8g, hydroxypropylmethylcellulose 4.0g, methylcellulose 1.3g, carbomer 0.1g, PEG400: 1.9g, sodium saccharin 0.1g.
[0030] The preparation method steps are as follows:
[0031] (1) Preparation of mosapride citrate solid dispersion: take mosapride citrate and hydroxypropyl cellulose and place them in a mixing agitator, add methanol, stir evenly to dissolve them; use a spray drier to dissolve the solution Prepare a solid dispersion, and finally get a mosapride citrate-hydroxypropyl cellulose solid dispersion;
[0032] (2) Preparation of an oral immediate-release film comprising mosapride citrate solid dispersion:
[0033] Disperse the hydroxypropyl methylcellulose evenly with an appropriate amou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com