Green environment-friendly synthetic method for mosapride citrate
A technology of mosapride citrate and a synthesis method, which is applied in the field of green environmental protection synthesis of mosapride citrate, can solve the problems of high manufacturing cost, complicated synthesis method, lengthy process and the like, and reduces the generation of three wastes The effect of improving the utilization rate of equipment and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
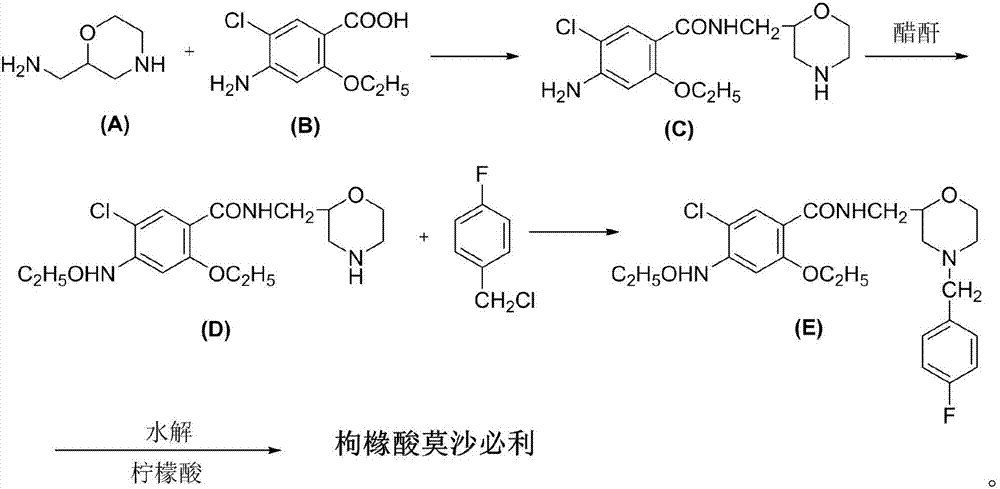
[0024] Intermediate (C): Preparation of 4-amino-5-chloro-2-ethoxy-N-(2-morpholinyl)methylbenzoic acid amine
[0025] Put 99g of 2-aminomethylmorpholine, 96g of triethylamine, 120g of isobutanol chloroformate and 3000ml of dichloromethane into the dry reaction flask, stir for 10min and cool to -10°C, slowly add 174g of 5-chloro-4- Amino-2-ethoxybenzoic acid, after the addition of -10~-5°C, the reaction was stirred at this temperature for 4h. After the reaction was finished, the reaction flask was washed several times with saturated brine, and the solvent dichloromethane was evaporated to dryness, 500 ml of absolute ethanol was added, the temperature was raised to dissolve, the crystals were refrigerated and filtered, and dried to obtain 231.3 g of intermediate (C), with a yield of 92 %.
[0026] Intermediate (D): Preparation of 4-acetylamino-5-chloro-2-ethoxy-N-(2-morpholinyl)methylbenzamide
[0027] 231.2g of intermediate (C), 200ml of acetic anhydride, and 100ml of glacial ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com