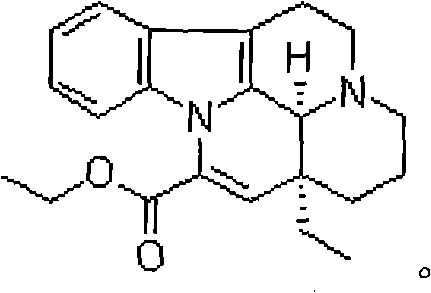
Medical composition
A technology of composition and medicine, applied in the field of liquid preparations containing vinpocetine and its preparation, which can solve the problem of no research report on vinpocetine injection, low quality of sterility assurance in the production process, and no high-temperature sterilization step and other problems, to achieve the effect of simplifying the composition of the prescription, improving the stability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
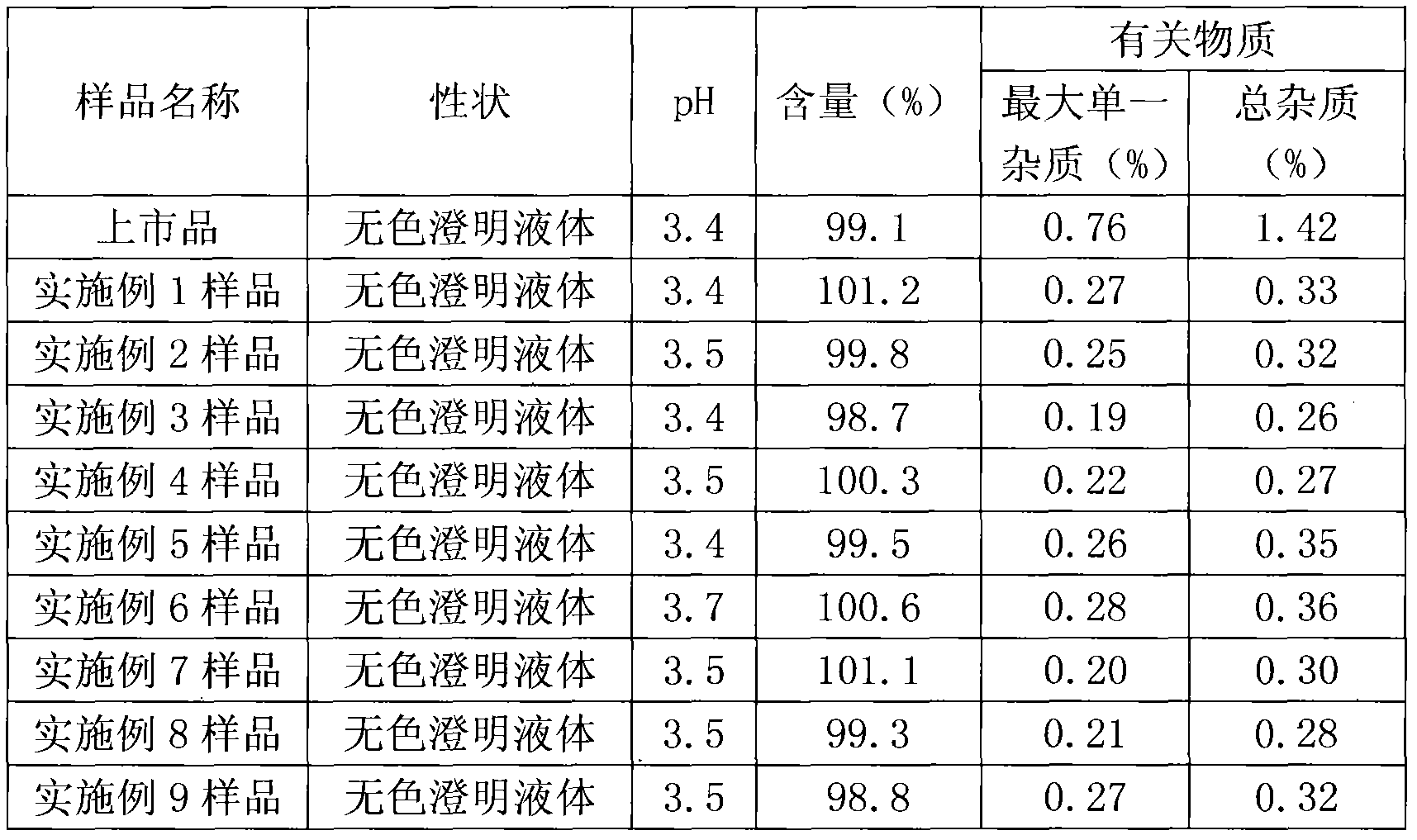
Embodiment 1
[0031] The prescription is as follows:
[0032] Vinpocetine 10g
[0033] Citric acid 5g
[0034] Water was added to 2000ml.
[0035] Preparation method: add citric acid into 80% water and stir to dissolve completely, add 1mol / l phosphoric acid to adjust pH to 2.2, add vinpocetine, stir to dissolve completely, add 0.1mol / l NaOH Adjust the pH of the aqueous solution to 3.4, add water to a sufficient amount, add injection-grade activated carbon (0.1%, w / v), insulate and stir at 60°C for 30 minutes, filter, fill, 2ml each, and sterilize at 121°C for 15 minutes. That is, Vinpocetine Injection.
Embodiment 2
[0037] The prescription is as follows:
[0038] Vinpocetine 10g
[0039] Citric acid 8g
[0040] Water was added to 2000ml.
[0041] Preparation method: add citric acid to 80% water and stir to dissolve it completely, add 1mol / l hydrochloric acid to adjust the pH to 2.0, add vinpocetine, stir to dissolve it completely, add 0.1mol / l NaOH Adjust the pH of the aqueous solution to 3.4, add water to a sufficient amount, add injection-grade activated carbon (0.1%, w / v), insulate and stir at 60°C for 30 minutes, filter, fill, 2ml each, and sterilize at 121°C for 15 minutes. That is, Vinpocetine Injection.
Embodiment 3
[0043] The prescription is as follows:
[0044] Vinpocetine 10g
[0045] Citric acid 10g
[0046] Water was added to 2000ml.
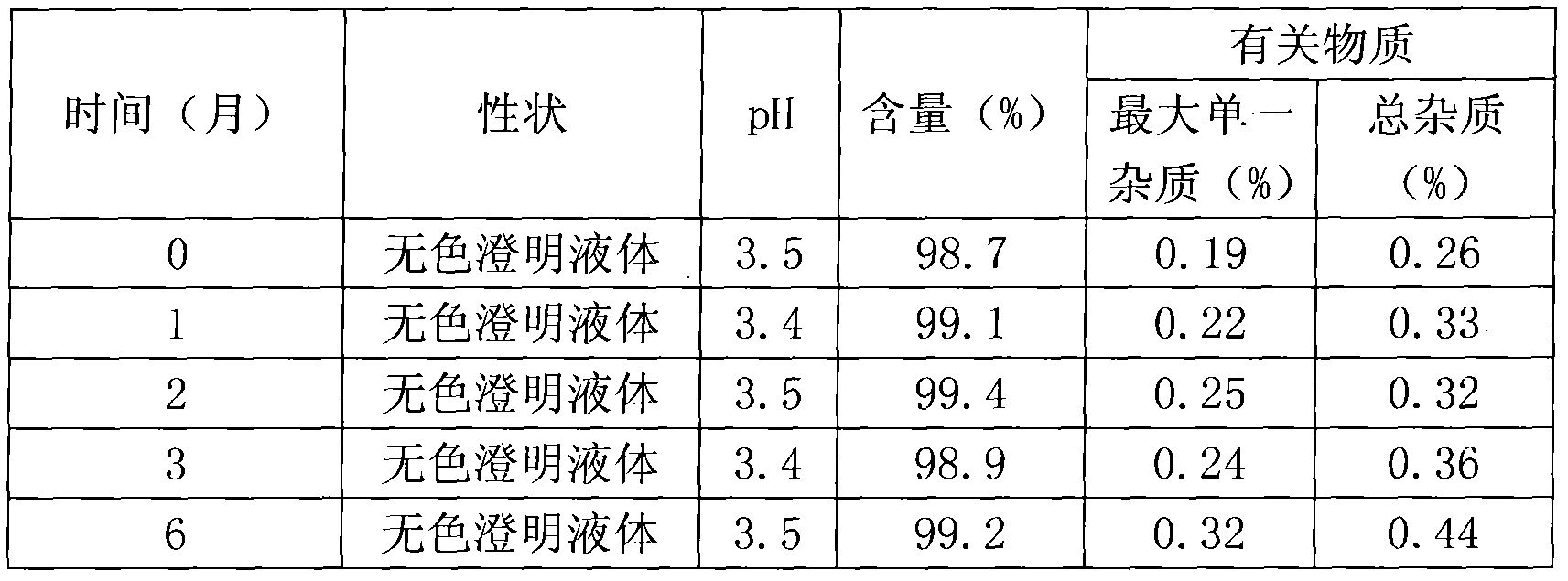
[0047] Preparation method: add citric acid to 80% water and stir to dissolve it completely, add vinpocetine, stir to dissolve it completely, add 0.1mol / l NaOH aqueous solution to adjust the pH to 3.4, add water to a sufficient amount , add injection-grade activated carbon (0.1%, w / v) at 60 DEG C and insulate and stir for adsorption for 30 minutes, filter, fill, each of 2ml, and sterilize at 121 DEG C for 12 minutes to obtain Vinpocetine Injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com