A kind of esomeprazole enteric-coated pellets and preparation method thereof
A technology for esomeprazole and pellets, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems of sudden release risks and side effects, and achieve no sudden release. risk, solving the risk of sudden release, the effect of clinical application safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Example 1: Preparation of Esomeprazole Magnesium Enteric-Coated Capsules
[0035]
[0036] Preparation Process:
[0037] 1. Prepare blank cores:
[0038] After the microcrystalline cellulose is crushed and passed through a 200 mesh sieve, part of it is added to the centrifugal pellet machine for mother use, and part of it is reserved for dusting; the starch is dissolved in an appropriate amount of hot purified water to prepare a starch with a concentration of 5% (g / ml) The slurry is used as a binder for standby; the centrifugal pellet machine is turned on to prepare pellets; after the pellets are raised, powder is added, and the pellets are enlarged according to the ratio of powder supply and liquid supply 2:1-3:1. , dried, and sieved to leave 35 mesh-45 mesh microcrystalline cellulose pellets for subsequent use.
[0039] 2. Calcium carbonate is pulverized to control the particle size of 0.5 μm-15 μm for later use; the crystal form stabilizer polyoxyethylene PEO a...
Embodiment 2
[0043] Embodiment two: the preparation of esomeprazole strontium dry suspension
[0044]
[0045] Preparation Process:
[0046] 1. Magnesium hydroxide is pulverized, and the particle size is controlled to be 0.5 μm-15 μm as a powder for later use. Polyoxyethylene PEO, polyvinyl pyrrolidone, and esomeprazole strontium are dissolved in 30% acetone solution to prepare a 10% (g / g) concentration, as a binder;
[0047] 2. Put the commercially available sugar pills into the centrifugal pill making machine, adopt the solid phase lamination process, spray the adhesive while dusting, control the centrifugal speed at 350-600 rpm, and the ratio of powder dusting and liquid spraying is 2:1: -3:1 for drug application, 30-40 ℃ blowing hot air to dry, to obtain drug-containing pellets.
[0048] 3. The product name "Opadry" gastric soluble coating powder is dissolved in the aqueous solution to prepare a solution of 8%-12% (g / g), and the core of the pill containing the drug is coated w...
Embodiment 3
[0068] Embodiment three: result analysis
[0069] 1. Stability comparison results:
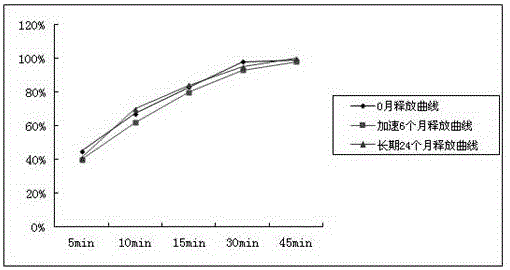
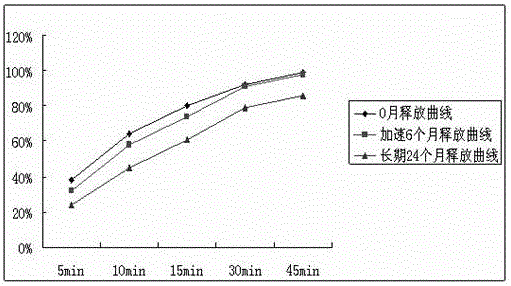
[0070] The samples prepared in Examples 1, 2 and Comparative Examples were respectively placed under the conditions of accelerated (temperature 40°C, humidity 75%) and long-term test (temperature 25°C, humidity 65%) according to the Chinese Pharmacopoeia method to detect product content, related Dissolution curves in substances and standard media, as follows:
[0071]
[0072] From the above experimental results, it can be clearly seen that the sample content and related substances prepared according to the technology provided by this patent are relatively stable during the stable storage period, and the release curve basically does not drift in 0 months, accelerated 6 months, and long-term 24 months.
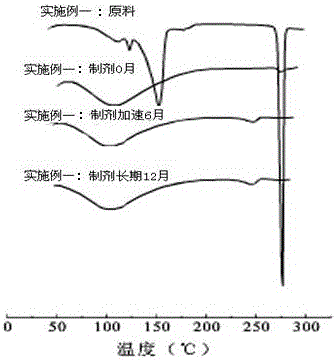
[0073] , DSC test comparison results:
[0074] Differential scanning calorimeter (DSC) is a technology that measures the energy difference between substances with temperature under program...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com