Enteric-coated tablet containing pantoprazole and preparation method thereof
A technology of pantoprazole and enteric-coated tablets, which is applied in the field of enteric-coated tablets containing pantoprazole and its preparation, can solve the problems of poor stability, cumbersome preparation process, decreased acid resistance and release rate, and achieve Ease of industrial production, simple production process, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
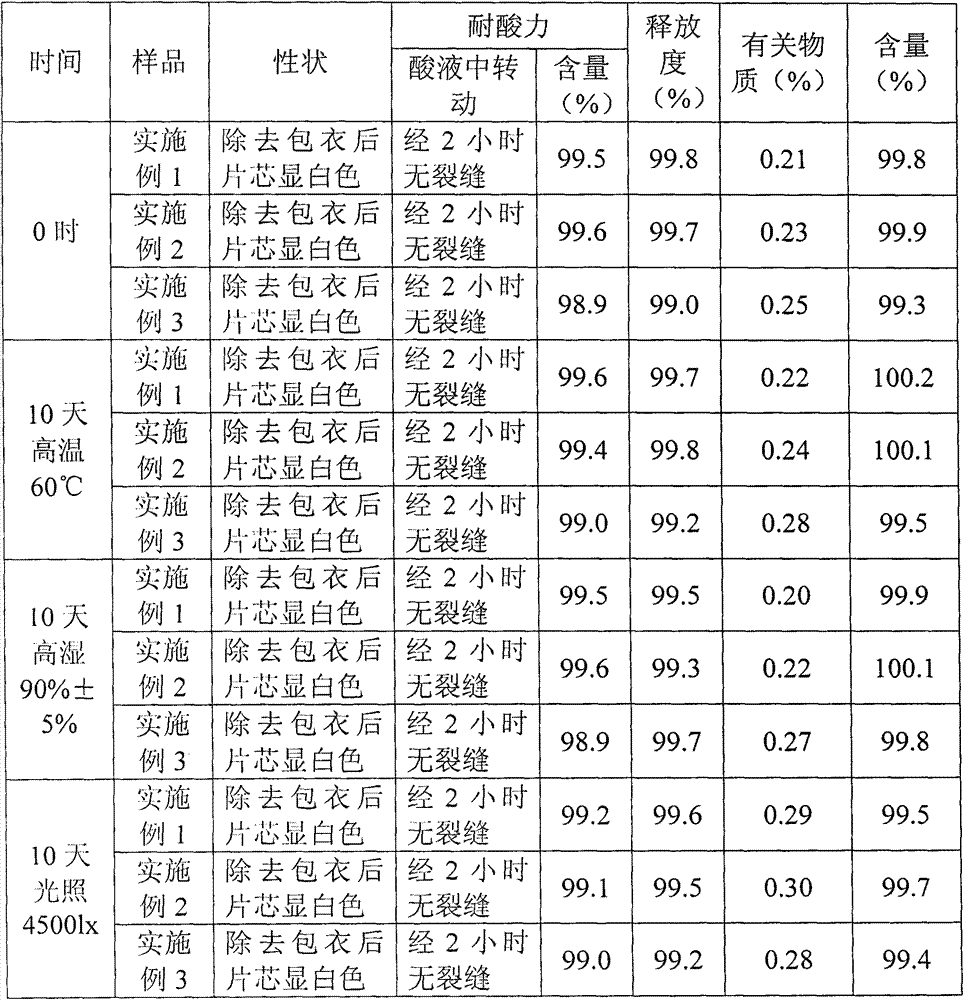
Embodiment 1
[0021] A kind of enteric-coated tablet containing pantoprazole
[0022] S11. Weigh 40 parts of pantoprazole; 20 parts of sodium carbonate; 1 part of sodium citrate; 0.1 part of vitamin E; 20 parts of filler, 1 part of lubricant; 5 parts of xylitol;
[0023] S12. Dissolve the sodium carbonate and sodium citrate weighed in step S11 in purified water and set aside
[0024] S13, dissolving the pantoprazole and vitamin E weighed in step S11 in absolute ethanol for subsequent use;
[0025] S14. Disperse the solution prepared in step S12 in the solution prepared in step S13 under stirring conditions, dry the mixed solution under reduced pressure, pulverize and sieve to obtain pantoprazole complex powder;
[0026] S15. Mix the powder obtained in step S14 with an appropriate amount of xylitol, a filler and a lubricant, and then directly compress the tablet to obtain a tablet core;
[0027] S16. Using hydroxypropyl methylcellulose as a film-forming material, polyethylene glycol as a p...
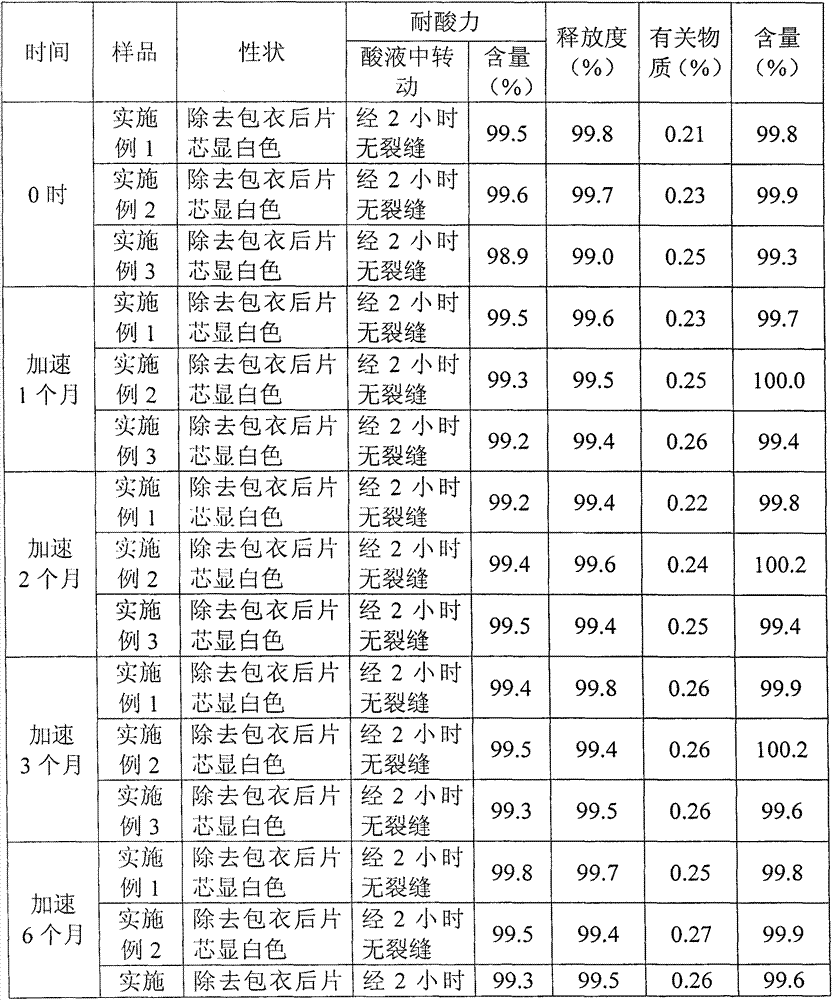
Embodiment 2
[0030] S21. Weigh 40 parts of pantoprazole sodium; 80 parts of sodium carbonate; 10 parts of sodium citrate; 1 part of vitamin E; 40 parts of filler, 5 parts of lubricant; 10 parts of xylitol;
[0031] S22. Dissolve the sodium carbonate and sodium citrate weighed in step S21 with purified water and set aside
[0032] S23, dissolving the pantoprazole and vitamin E weighed in step S21 in absolute ethanol for subsequent use;
[0033] S24. Disperse the solution prepared in step S22 in the solution prepared in step S23 under stirring conditions, dry the mixed solution under reduced pressure, pulverize and sieve to obtain pantoprazole complex powder;
[0034] S25, mixing the powder obtained in step S24 with an appropriate amount of xylitol, a filler and a lubricant, and then directly compressing the tablet to obtain a tablet core;
[0035] S26, using hydroxypropyl methylcellulose as film-forming material, polyethylene glycol as plasticizer, talcum powder as anti-adhesive agent, wat...
Embodiment 3
[0038] S31. Weigh 40 parts of pantoprazole; 50 parts of sodium carbonate; 5.5 parts of sodium citrate; 0.55 parts of vitamin E; 30 parts of filler, 3 parts of lubricant; 7.5 parts of xylitol;
[0039] S32, dissolving the sodium carbonate and sodium citrate weighed in step S31 with purified water, and set aside
[0040] S33, dissolving the pantoprazole and vitamin E weighed in step S31 in absolute ethanol for subsequent use;
[0041] S34. Disperse the solution prepared in step S32 in the solution prepared in step S33 under stirring conditions, dry the mixed solution under reduced pressure, pulverize and sieve to obtain pantoprazole complex powder;
[0042] S35, mixing the powder obtained in step S34 with an appropriate amount of xylitol, a filler and a lubricant, and then directly compressing into tablets to obtain tablet cores;
[0043] S36. Using hydroxypropyl methylcellulose as a film-forming material, polyethylene glycol as a plasticizer, talcum powder as an anti-adhesive ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com