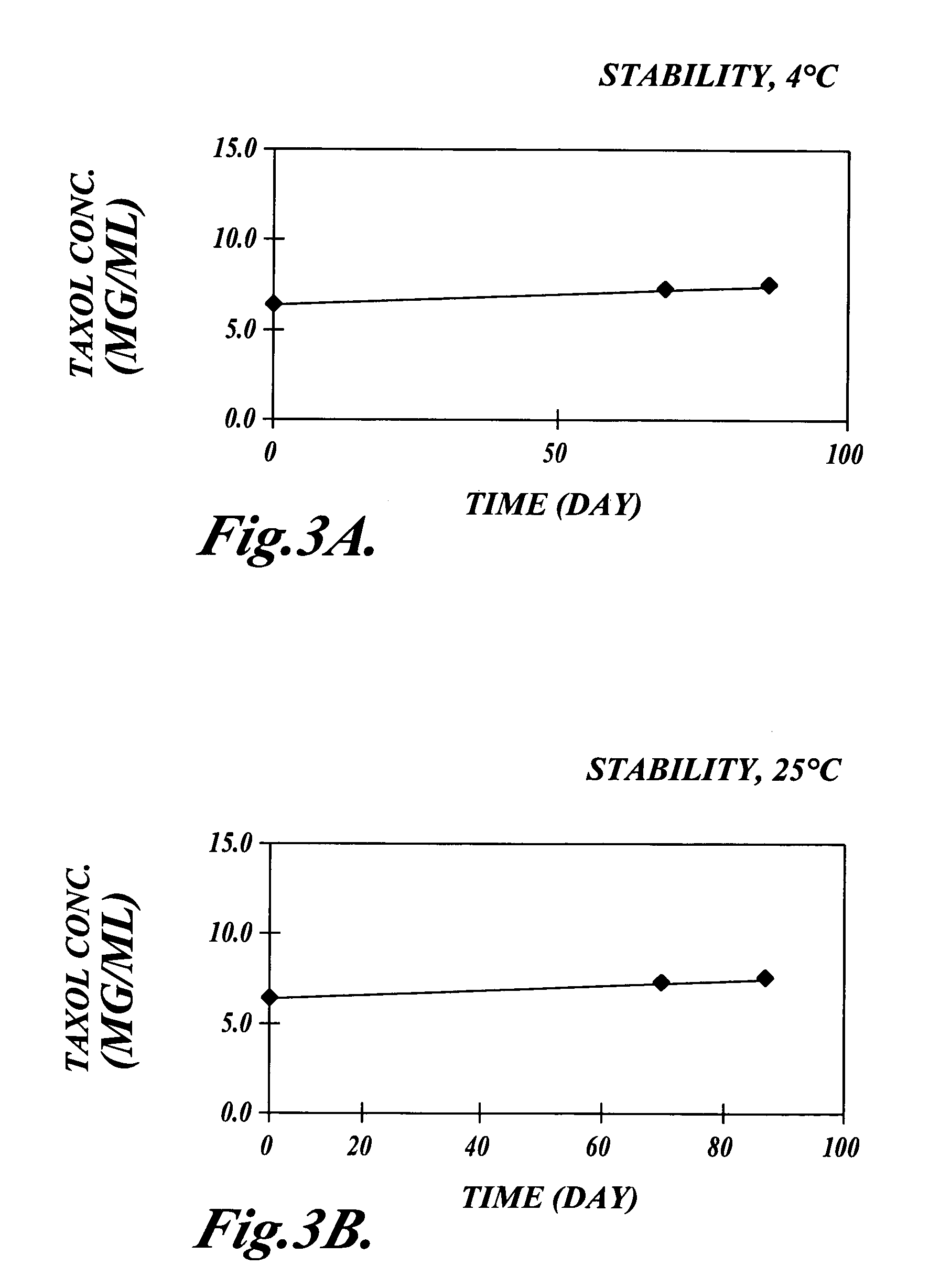
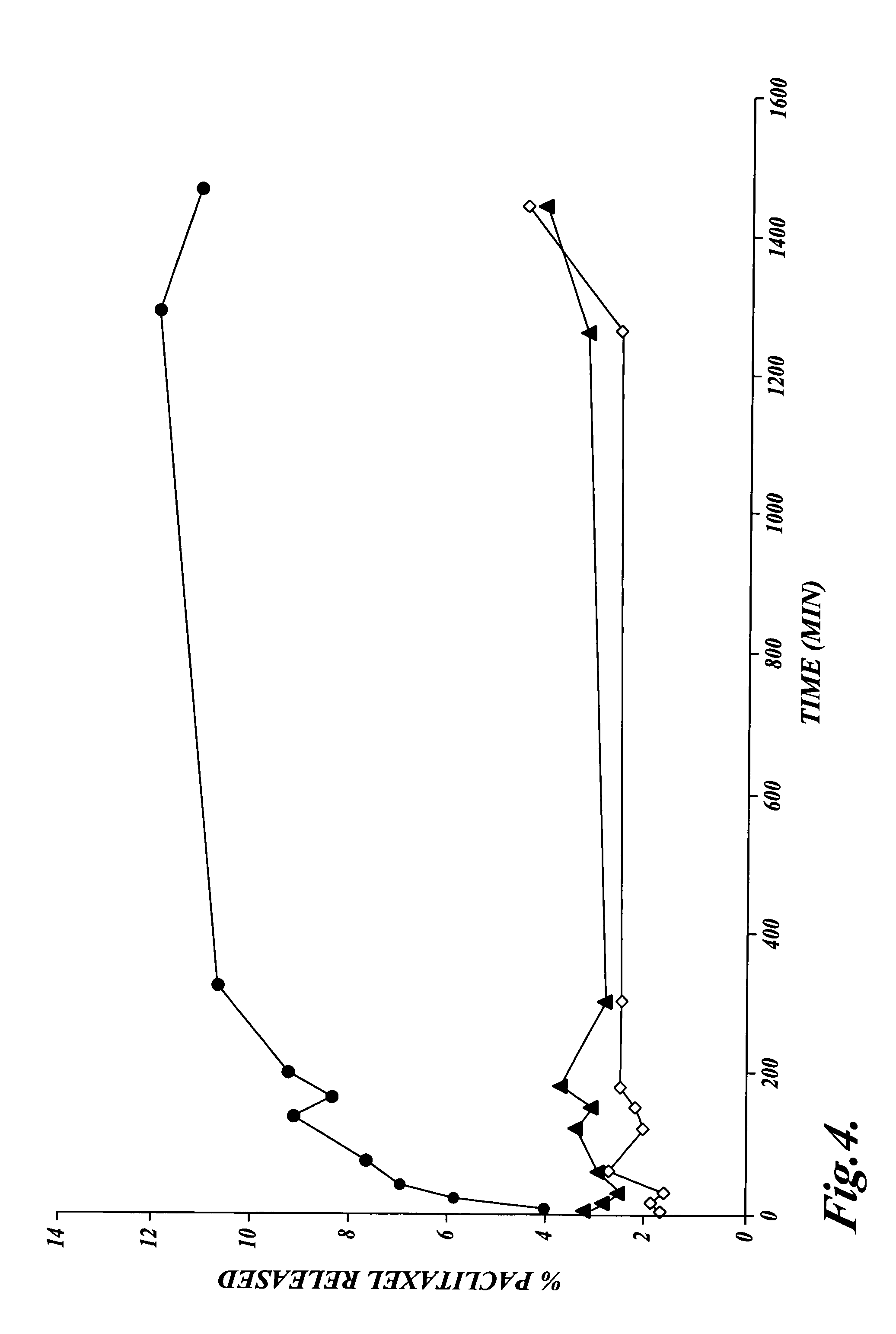
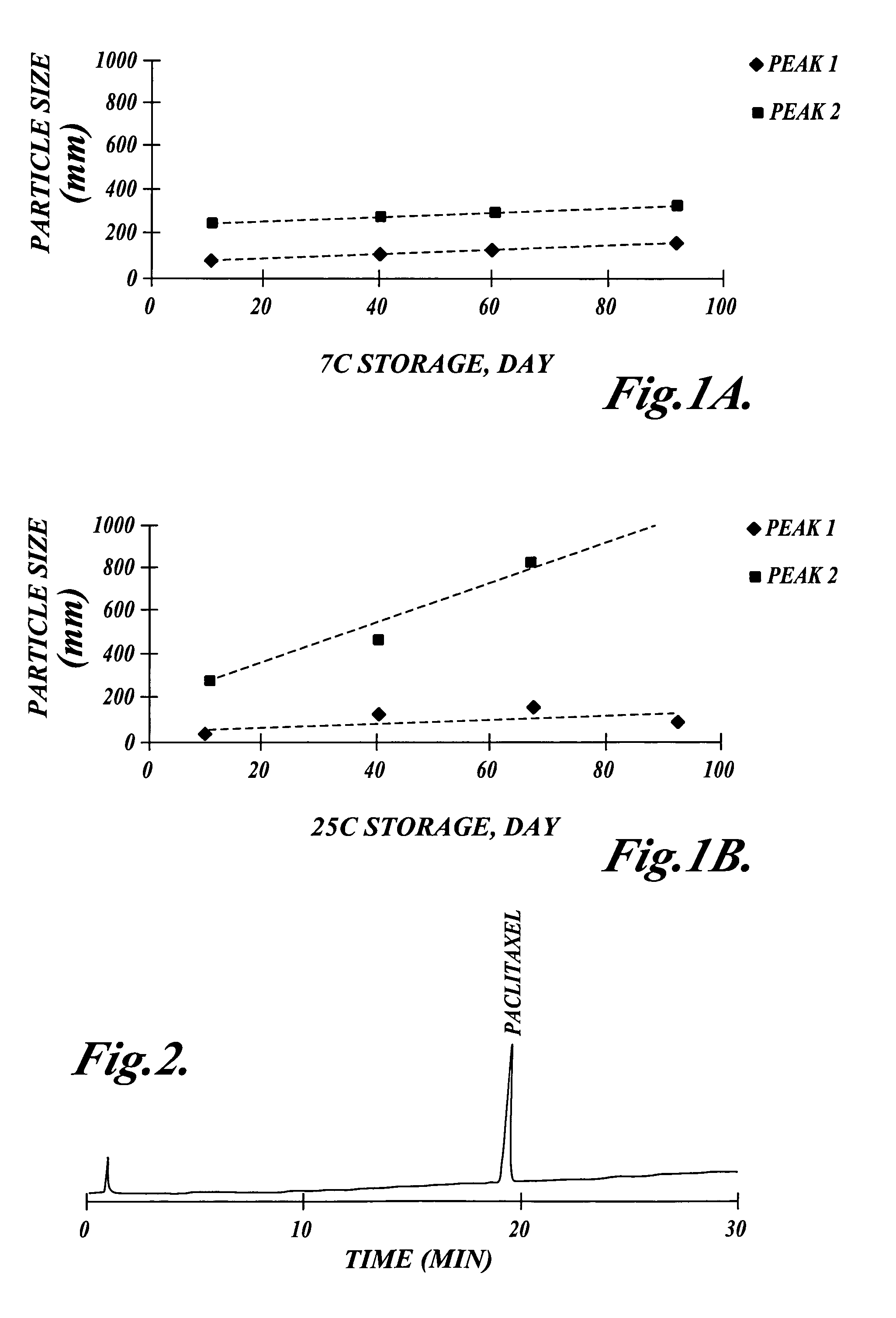
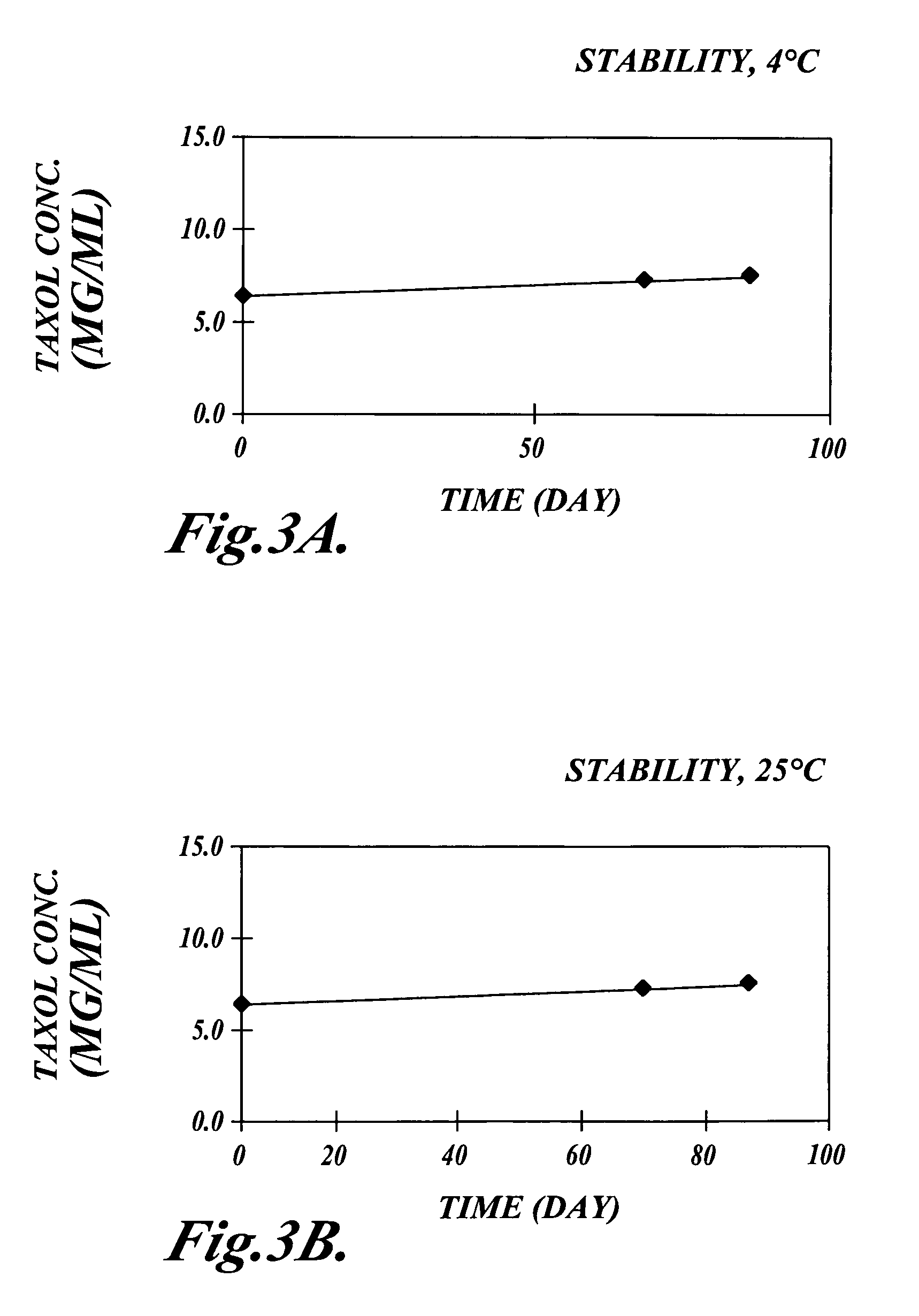
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
49 results about "Tocophersolan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
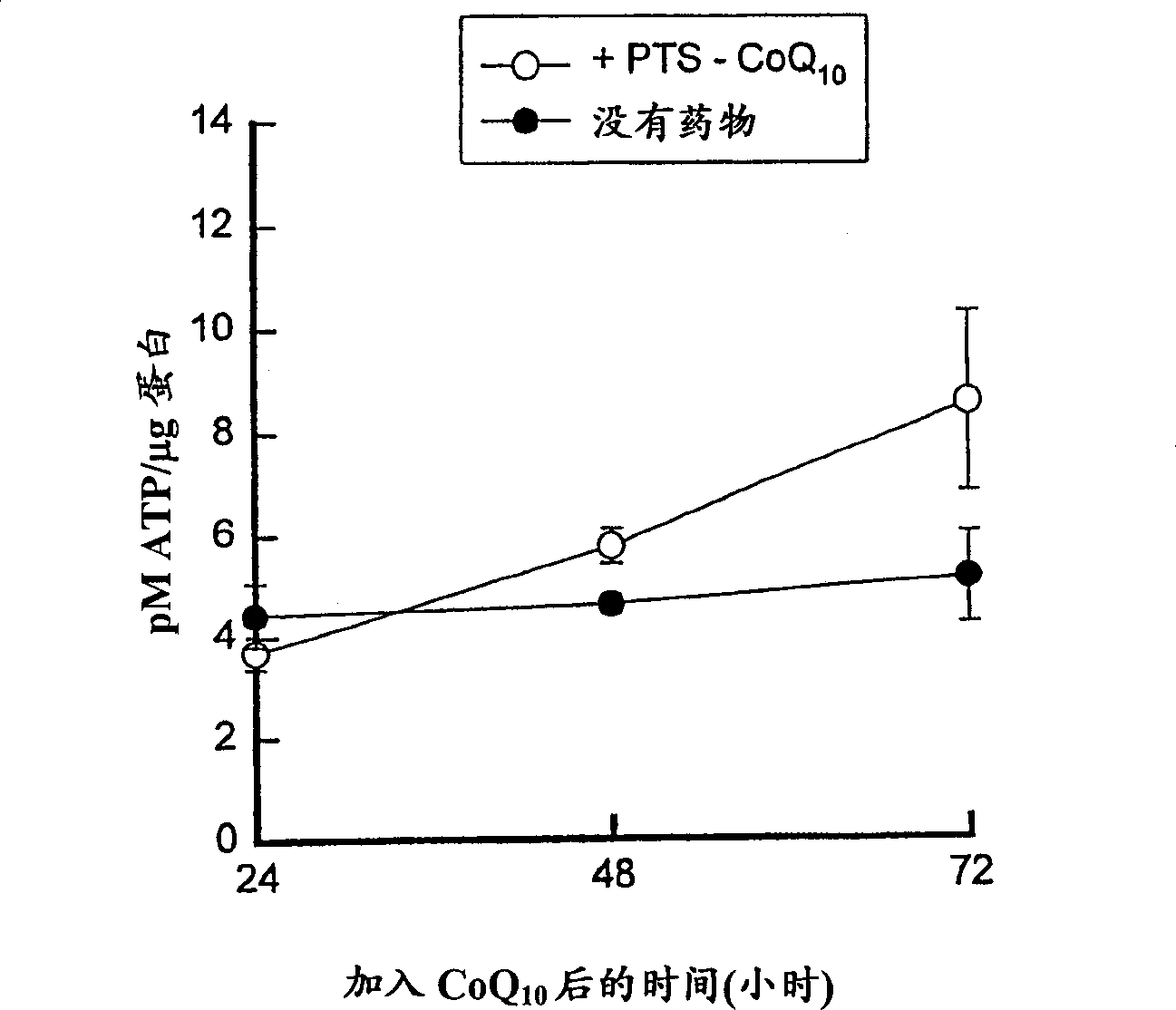
A water-soluble amphipathic formulation of d-alpha-tocopherol succinate coupled, through a succinate linker, to polyethylene glycol (PEG) 1000. Due to its amphipathic property in which it forms its own micelles, tocophersolan is easily taken up into enterocytes, even in the absence of bile salts; fat soluble d-alpha-tocopherol is then released after hydrolysis. This formulation enhances the absorption of d-alpha-tocopherol compared to the administration of free d-alpha-tocopherol. In addition, tocophersolan may enhance the absorption of water-insoluble agents and other fat-soluble vitamins.
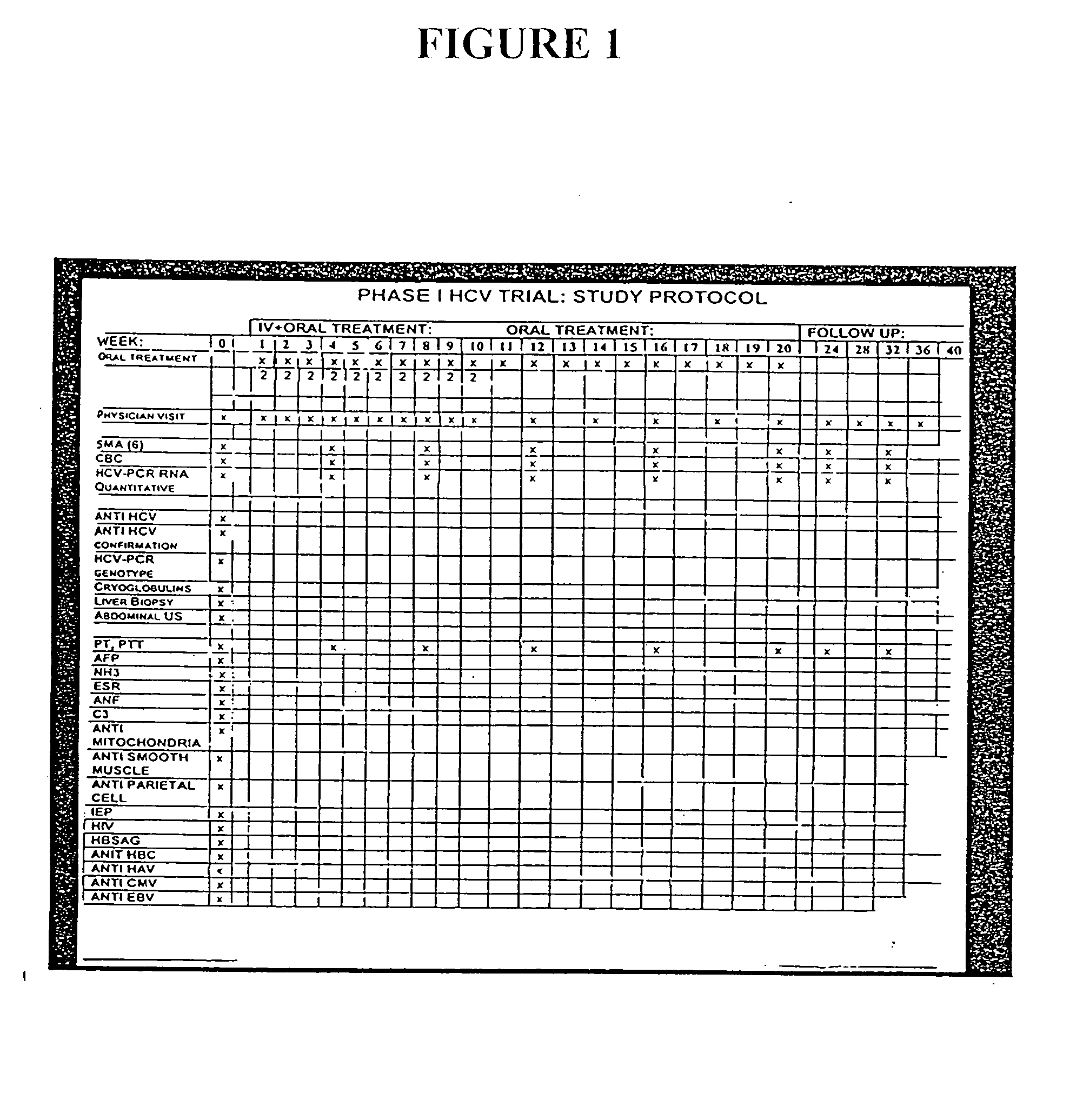
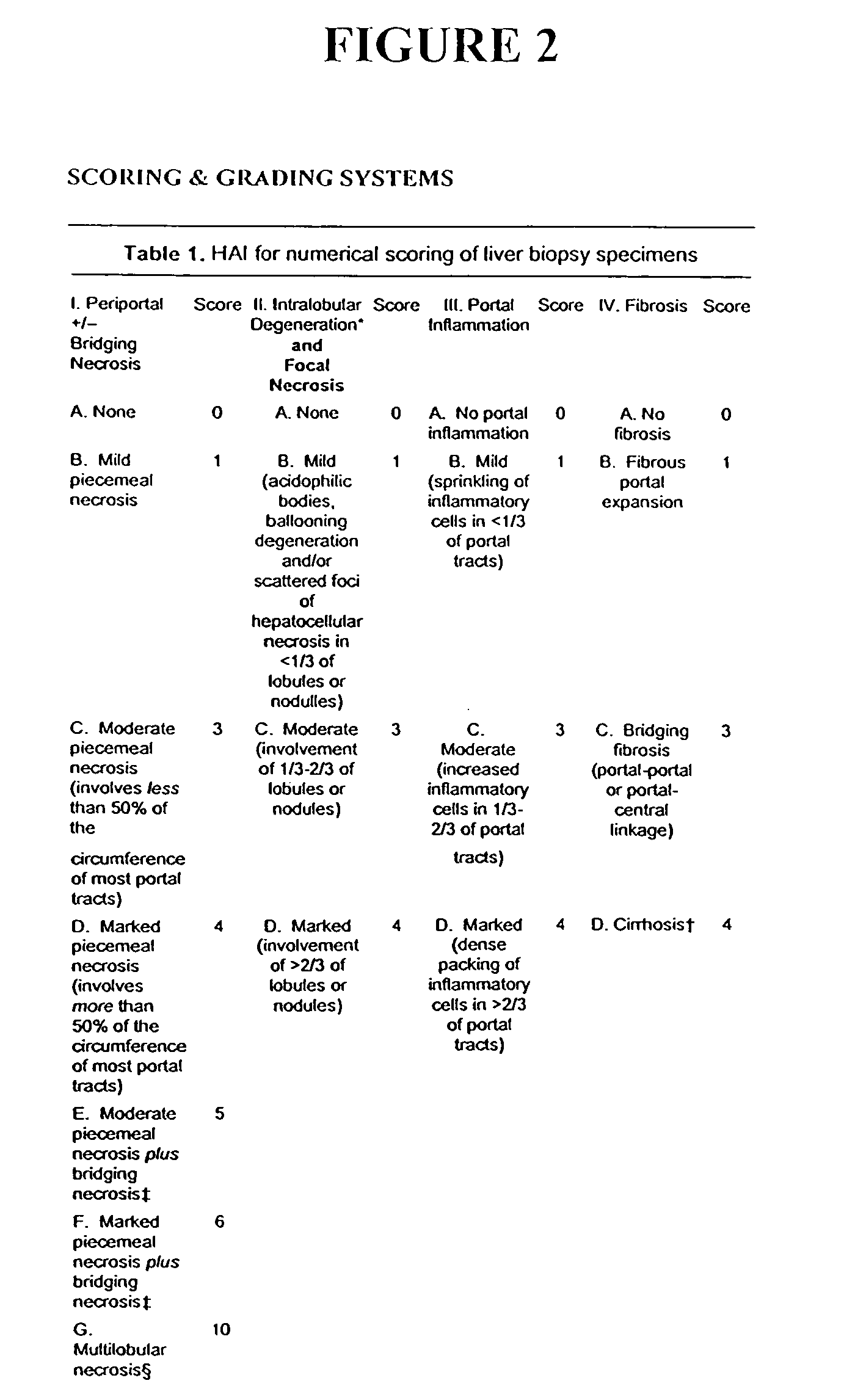
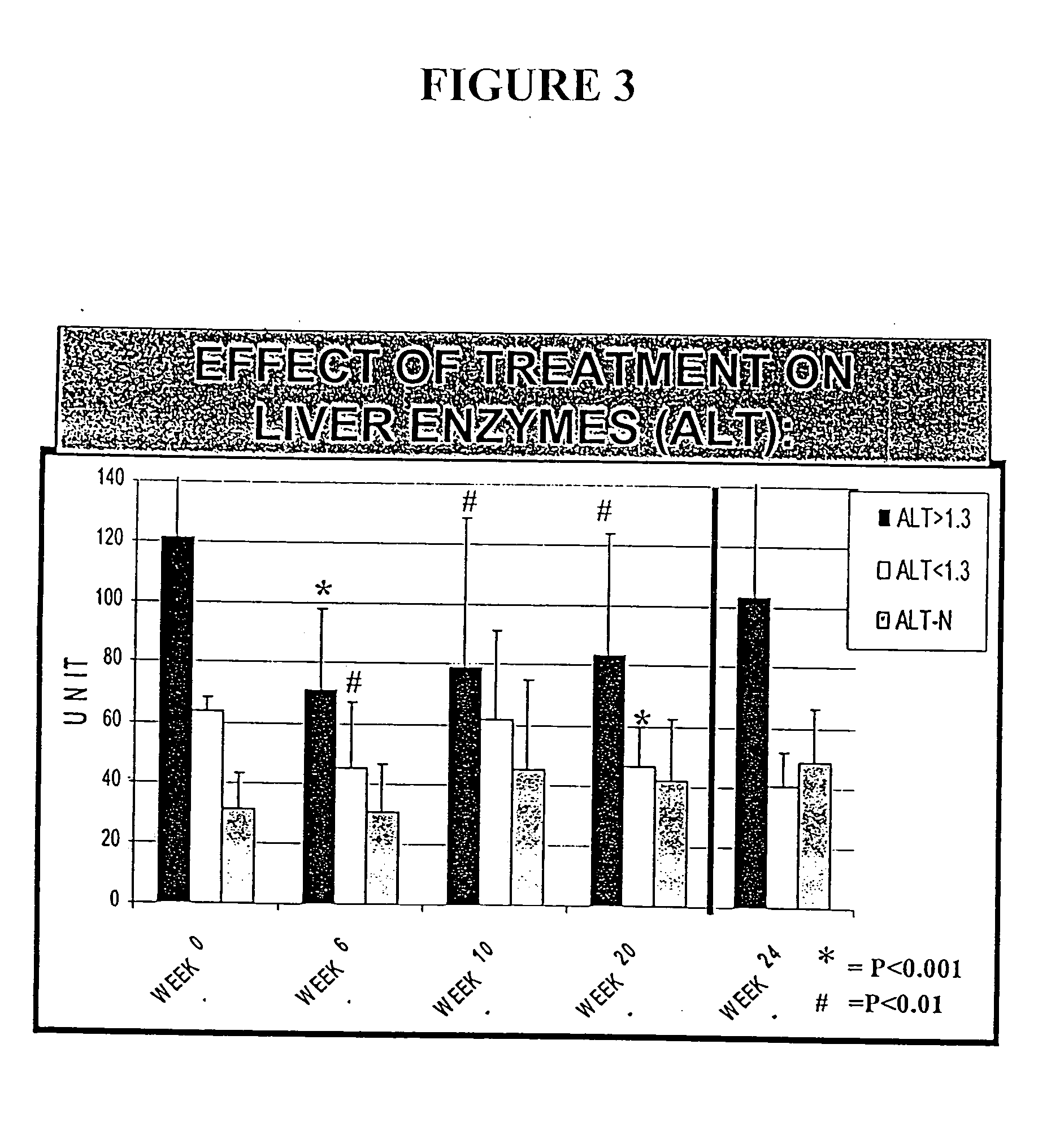
Compositions and methods useful for treating and preventing chronic liver disease, chronic HCV infection and non-alcoholic steatohepatitis
InactiveUS20050123628A1Reduce oxidative stressReduce lipid peroxidationBiocideDipeptide ingredientsChronic viral hepatitis CLipid peroxidation
The invention relates generally to compositions comprising antioxidants useful for reducing oxidative stress and lipid peroxidation, and treating chronic liver disease, chronic hepatitis C virus infection and non-alcoholic steatohepatitis. In particular, the invention relates to the preparation and oral administration of compositions comprising glycyrrhizin, schisandra, ascorbic acid, L-glutathione, silymarin, lipoic acid, and d-alpha-tocopherol. The invention also relates to the preparation and parenteral administration of compositions comprising glycyrrhizin, ascorbic acid, L-glutathione, and vitamin B-complex, preferably by infusion or intravenous injection. The invention further relates to methods of using the antioxidants, oral compositions and parenteral compositions.
Owner:ZABRECKY GEORGE
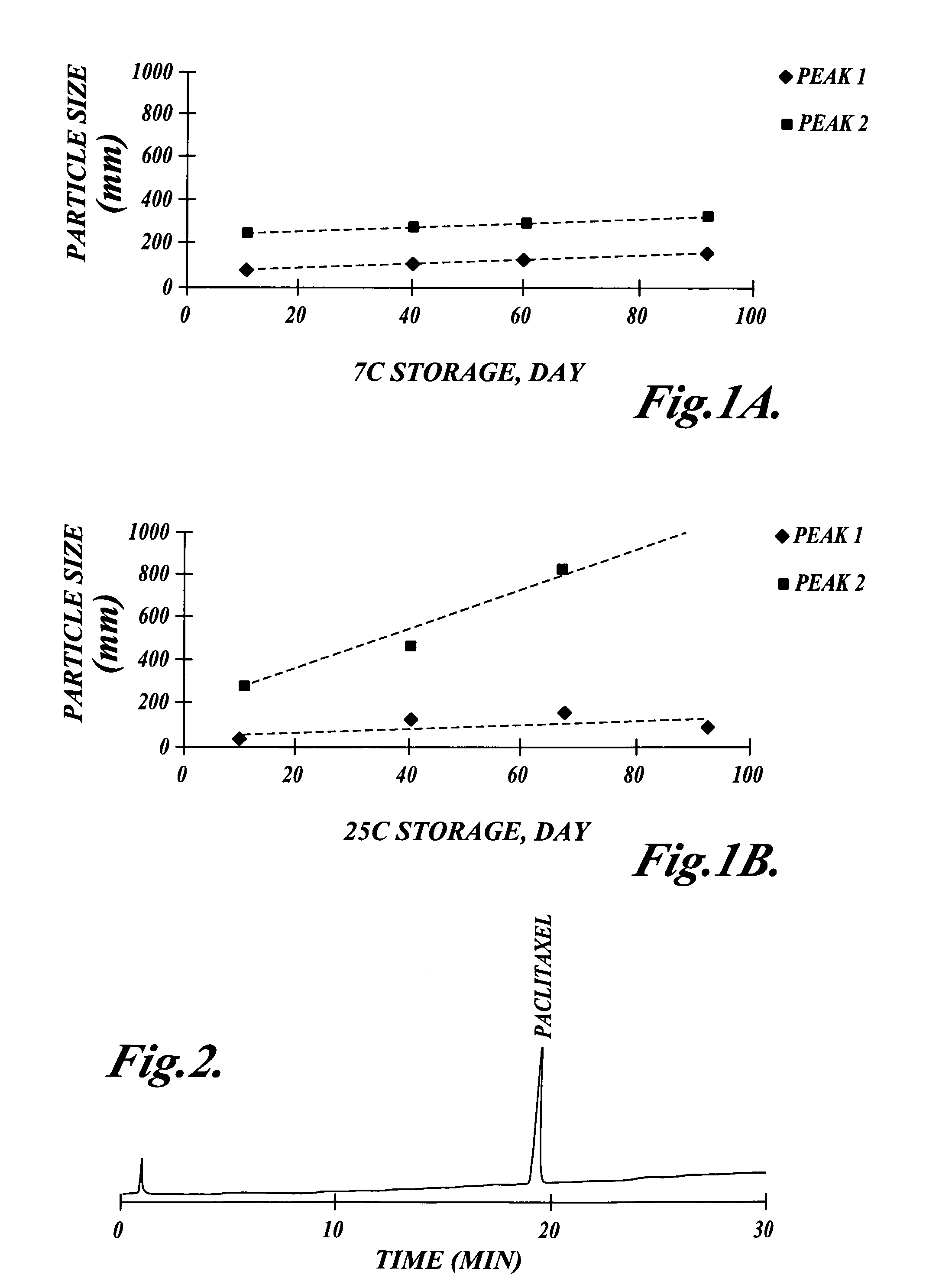
Emulsion vehicle for poorly soluble drugs
An emulsion of α-tocopherol, stabilized by biocompatible surfactants, as a vehicle or carrier for therapeutic drugs, which is substantially ethanol free and which can be administered to animals or humans via various routes is disclosed. Also included in the emulsion is PEGylated vitamin E. PEGylated α-tocopherol includes polyethylene glycol subunits attached by a succinic acid diester at the ring hydroxyl of vitamin E and serves as a primary surfactant, stabilizer and a secondary solvent in emulsions of α-tocopherol.
Owner:IGDRASOL
Emulsion vehicle for poorly soluble drugs
InactiveUS7030155B2Process stabilityEmulsion stabilizationOrganic active ingredientsBiocideEmulsionSuccinic acid
A method of making an emulsion of tocopherol incorporating a co-solvent and, stabilized by biocompatible surfactants, as a vehicle or carrier for therapeutic drugs, which is substantially ethanol free and which can be administered to animals or humans by various routes, is disclosed. Also included in the emulsion is PEGylated vitamin E. PEGylated α-tocopherol includes polyethylene glycol subunits attached by a succinic acid diester at the ring hydroxyl of vitamin E and serves as a primary surfactant, stabilizer and a secondary solvent in emulsions of α-tocopherol.
Owner:IGDRASOL
Method of preventing flavor component from degradation
InactiveUS20050287259A1Appearance be impairedTaste be impairedFruit and vegetables preservationMilk preservationFlavorAlcohol
The present invention relates to a deterioration preventive for a flavor component, which is an oil-in-water and / or -polyhydric alcohol type emulsion, comprising an extracted tocopherol, wherein d-δ-tocopherol is contained in an amount of 45% by weight or more of a total tocopherol, ferulic acid and / or a derivative thereof, and a polyglycerol fatty acid ester; a deterioration preventive for a flavor component, which is an oil-in-water and / or -polyhydric alcohol type emulsion, comprising (A) the above-mentioned extracted tocopherol, (B) ferulic acid and / or a derivative thereof, and (C) an emulsifying agent having an HLB of 9 or more; a flavor for foodstuff, comprising the above-mentioned deterioration preventive; an emulsion flavor for foodstuff, comprising the above-mentioned extracted tocopherol, a catechin; and a polyglycerol fatty acid ester; and foodstuff comprising the above-mentioned deterioration preventive for a flavor component, the above-mentioned flavor for foodstuff, or the above-mentioned emulsion flavor for foodstuff.
Owner:TAIYO KAGAKU CO LTD
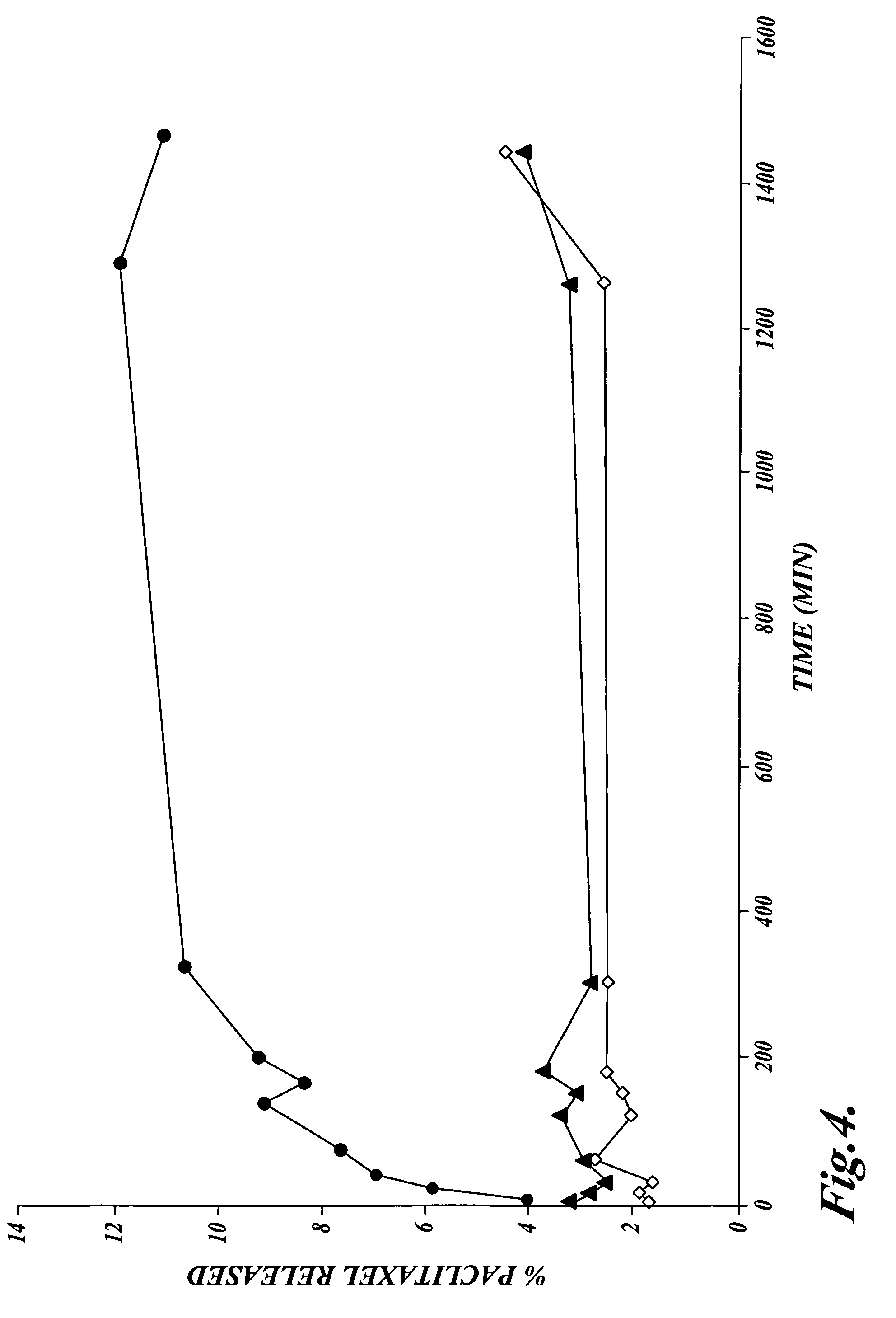
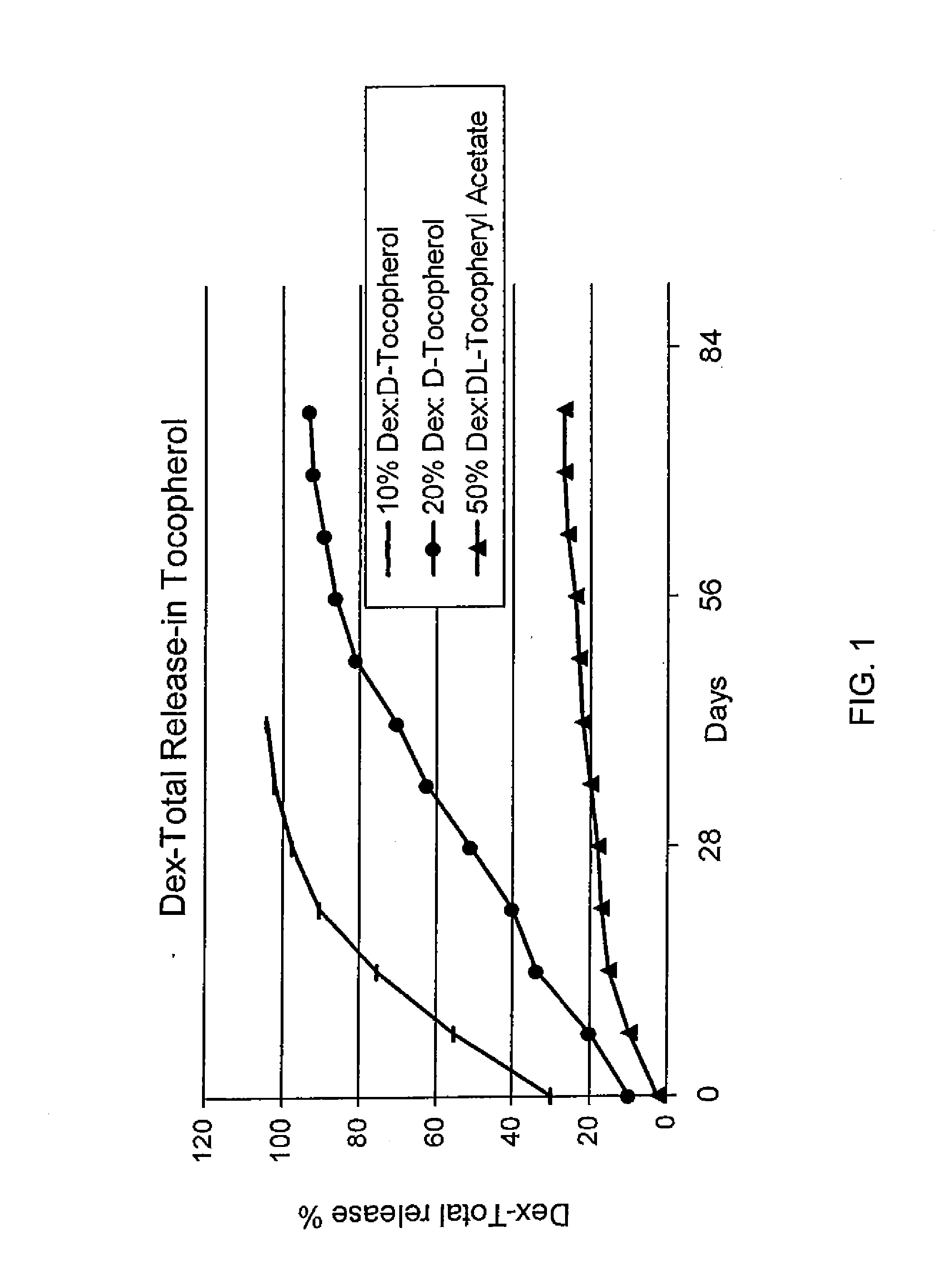
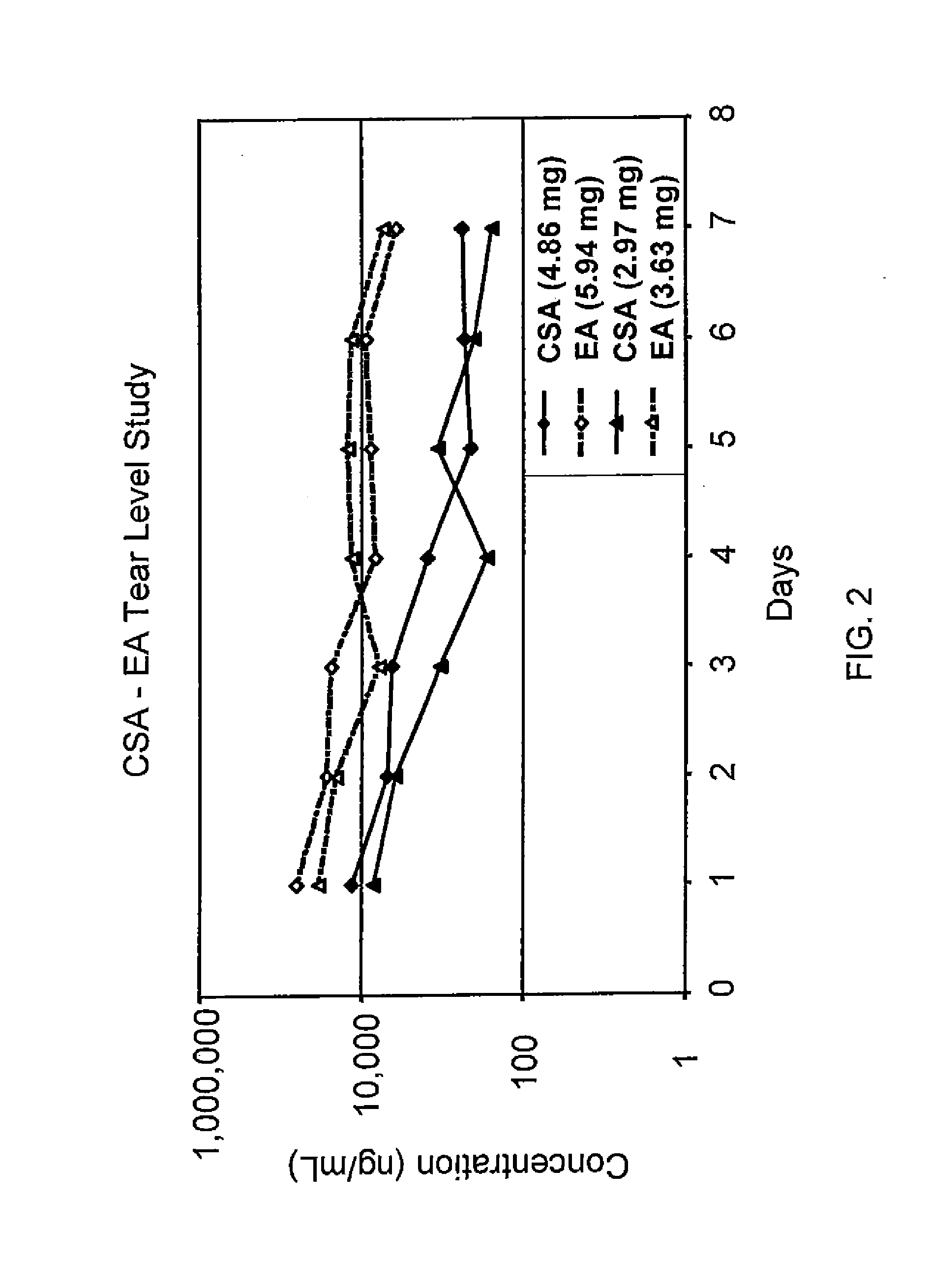
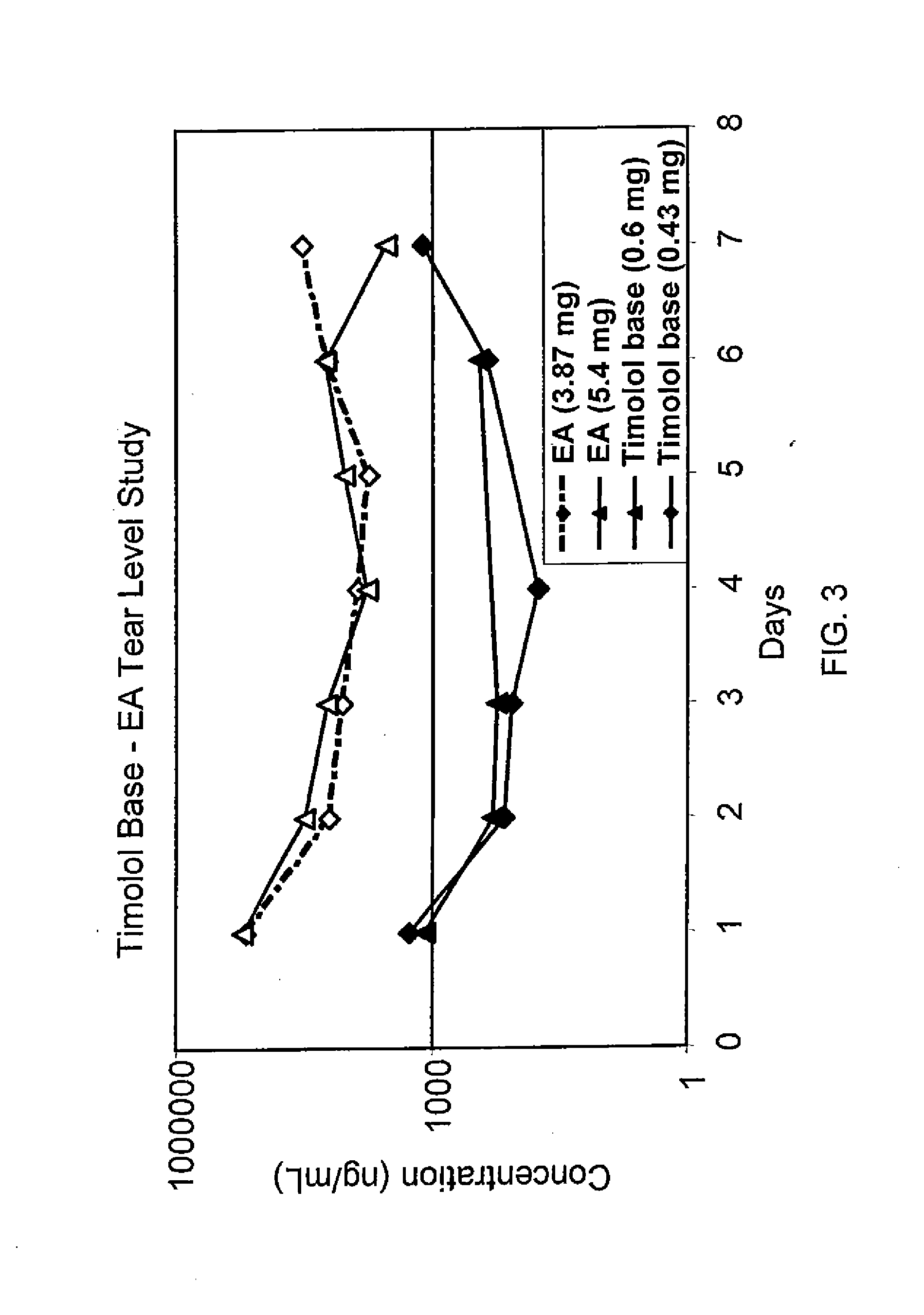
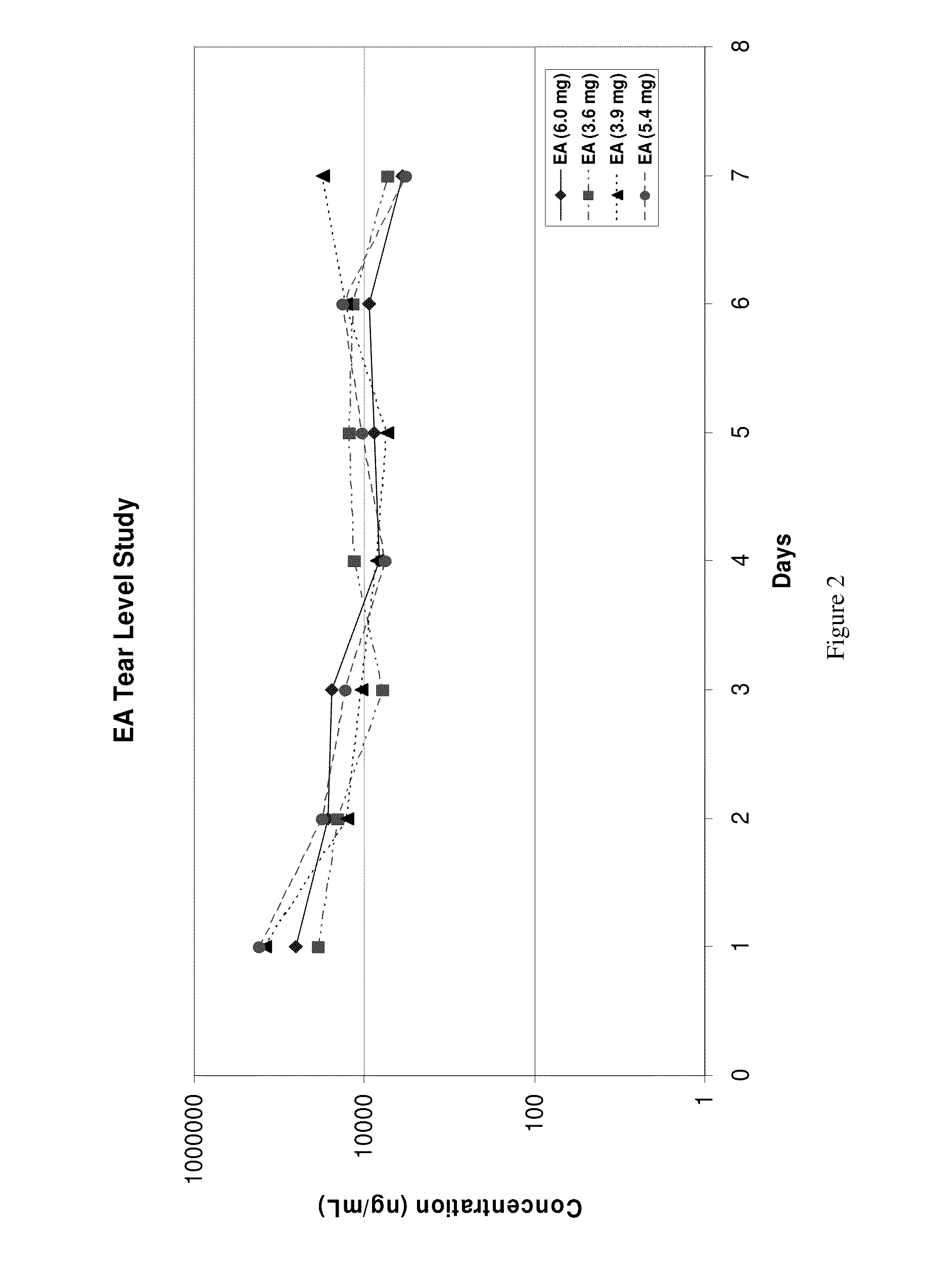
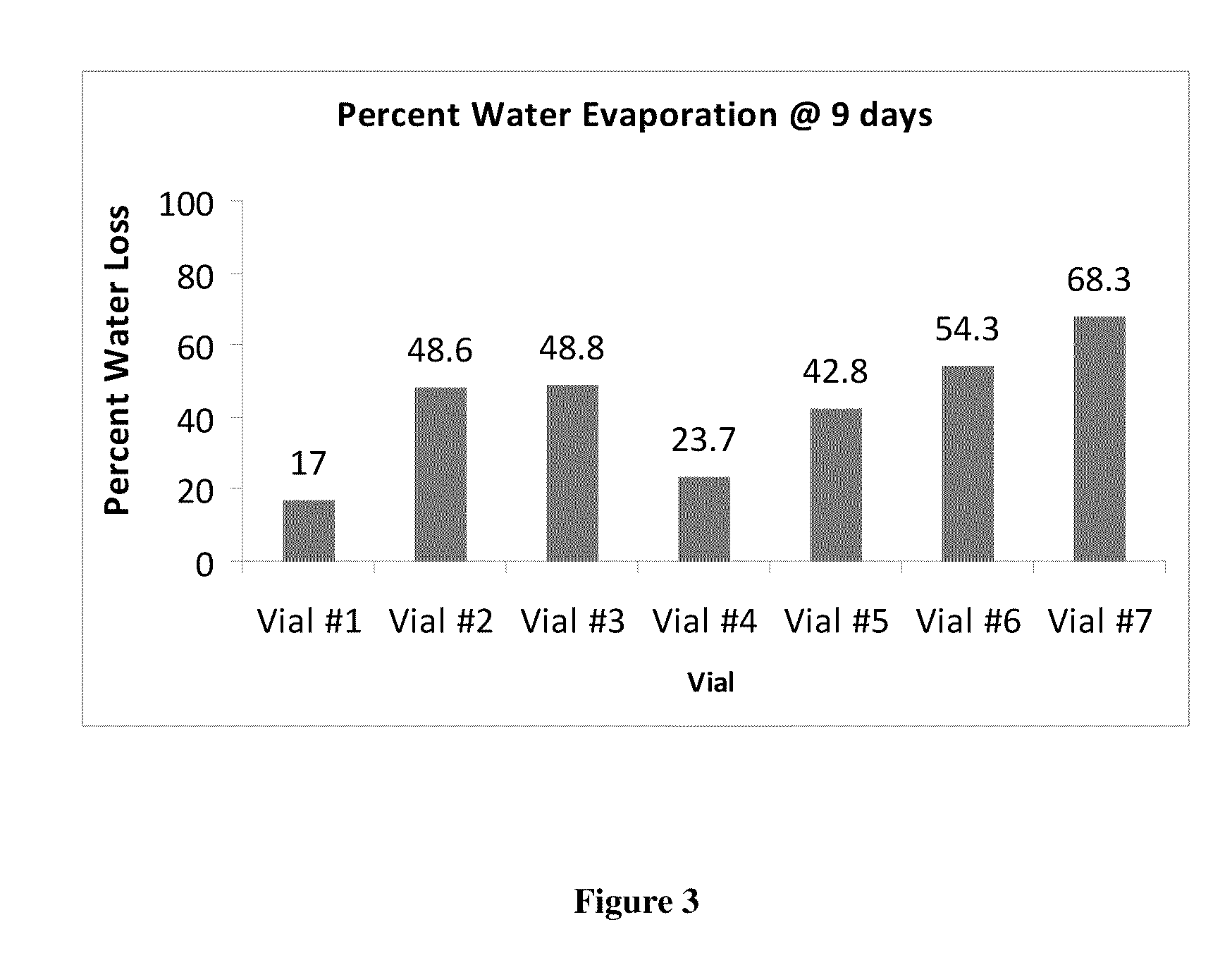
Sustained release eye drop formulations
ActiveUS20130324481A1Easy to useEfficient deliveryBiocideTetracycline active ingredientsSolubilityEye/ear drops
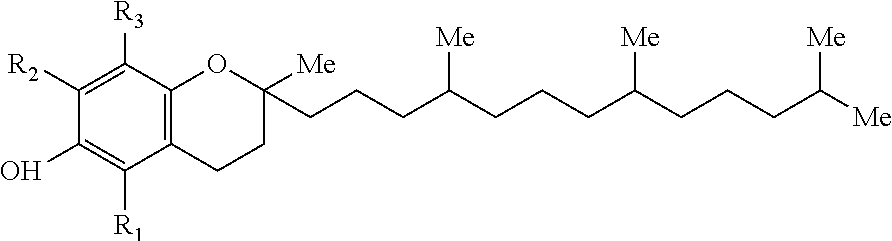
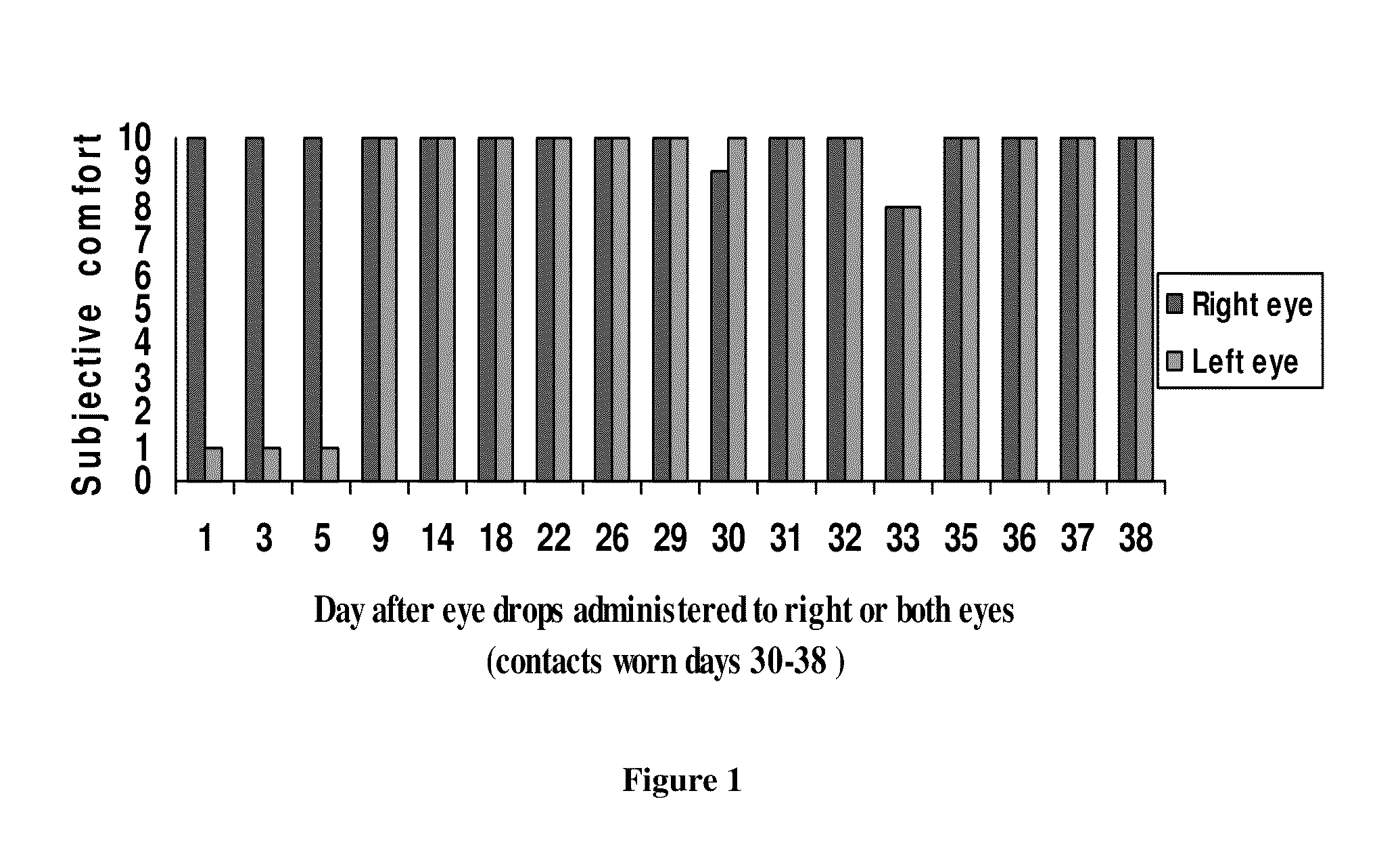
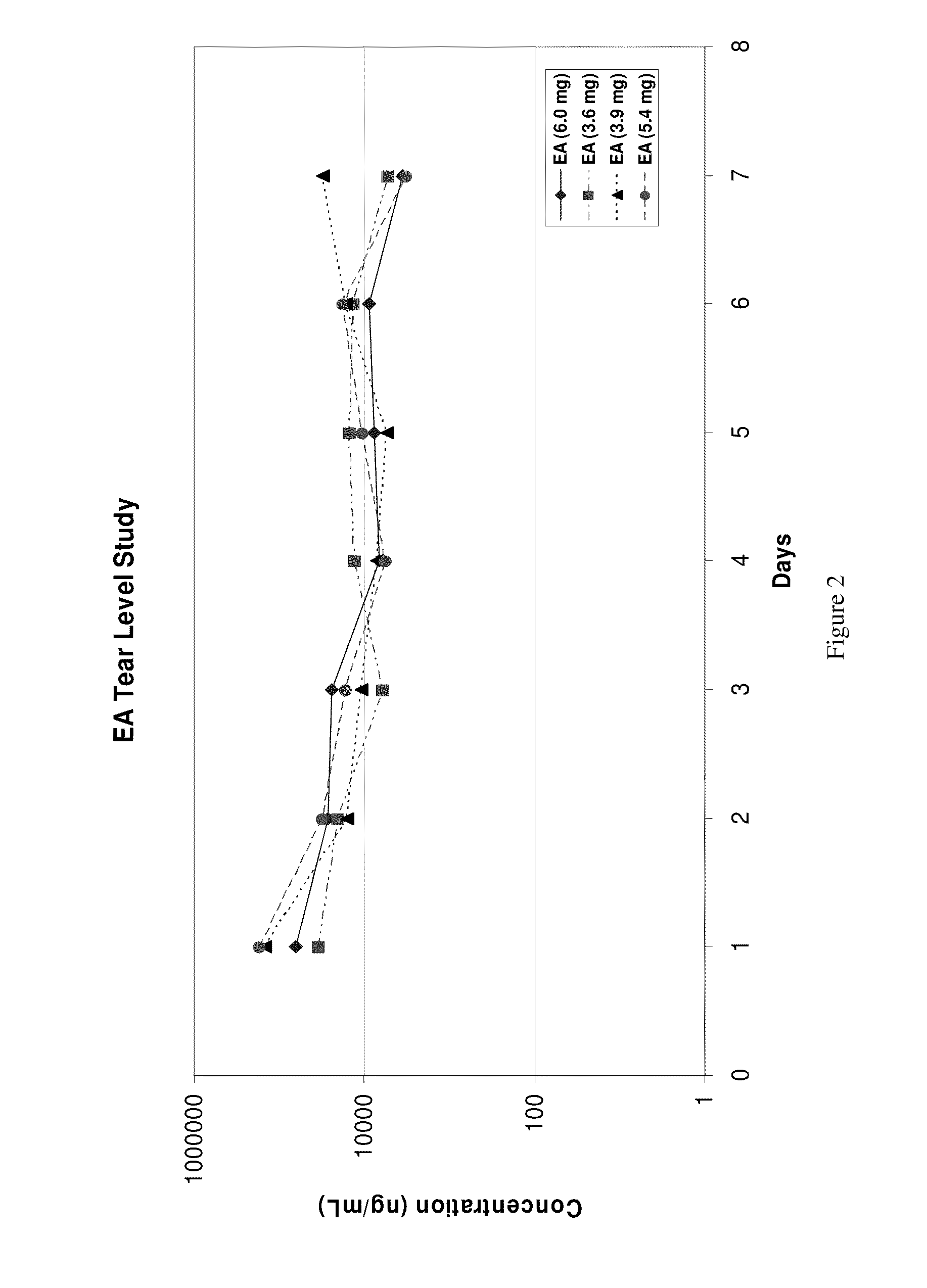
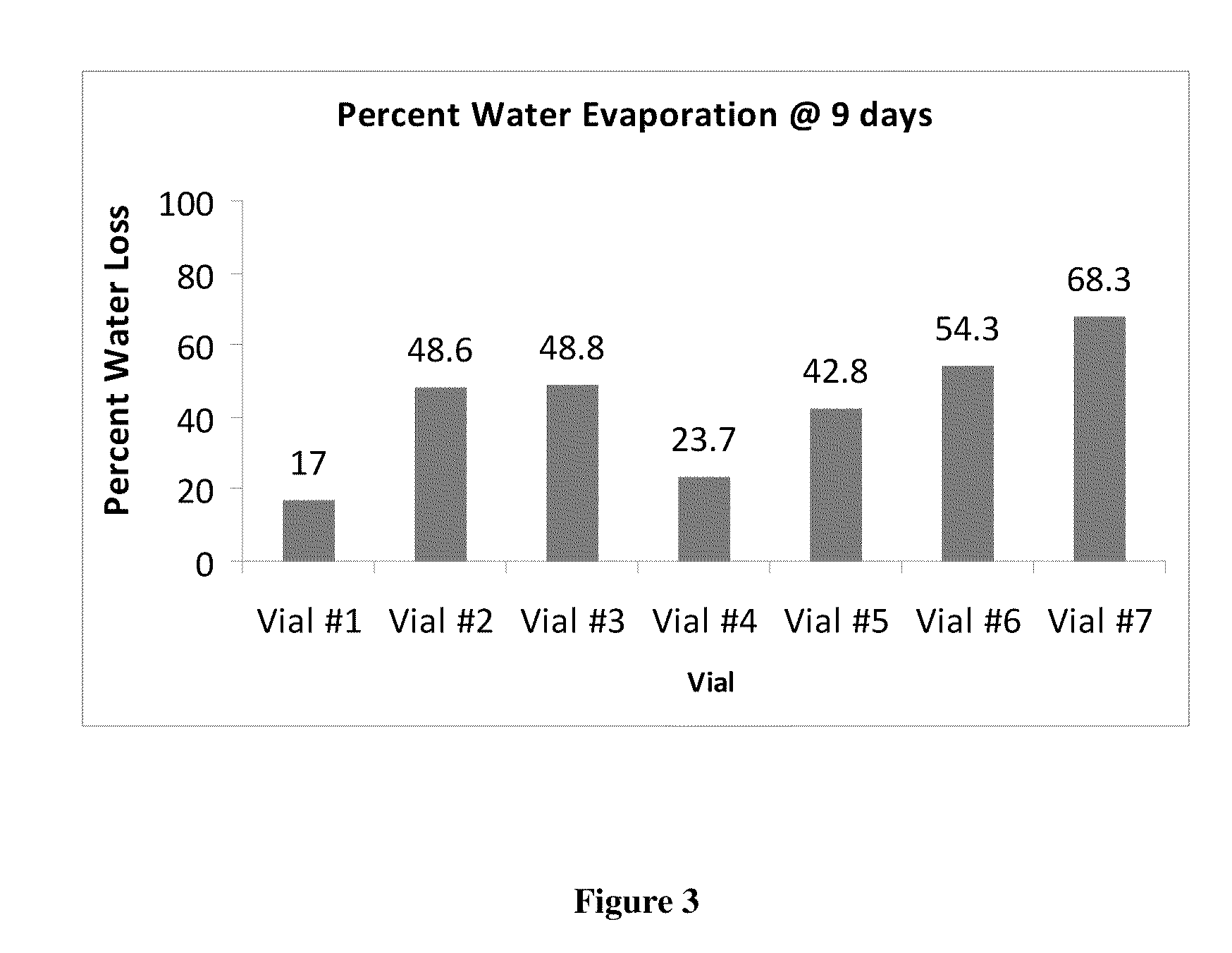
This invention provides for biocompatible, biodegradable eye drop pharmaceutical formulations useful for the treatment of ocular indications. In particular, tocopherols and their esters of low water solubility, notably α-tocopheryl acetate, are exceptional vehicles for biocompatible, nonirritating topical eye drop formulations that provide sustained release of active agents.
Owner:RAMSCOR
Formulation and delivery method to enhance antioxidant potency of Vitamin E
InactiveUS7012092B2Improve antioxidant capacityGood synergyOrganic active ingredientsBiocideDiseaseOxygen radical absorbance capacity
A formulation to deliver a full-spectrum of Vitamin E isomers for improved antioxidant capacity, bioavailability, dissolution and efficacy. The formulation includes dl-α-tocopheryl acetate or dl-α-tocopheryl succinate (synthetic Vitamin E), natural Vitamin E and mixed tocopherols, such as α-, β-, γ- and δ-tocopherol, as well as four isomers (α, β, γ and δ) of tocotrienols. This formulation is designed to deliver at least 17-times the antioxidant capacity of synthetic Vitamin E (dl-α-tocopheryl acetate), and at least twice the antioxidant capacity of natural Vitamin E (d-α-tocopherol) as measured by oxygen radical absorbance capacity (ORAC) assay. The potent antioxidant capacity of this formula affords protection against oxidative damage of cell membranes, heart disease, cancer and eye and skin disease.
Owner:RICH MEL +2
Compositions and methods for treating lung diseases and lung injury
InactiveUS20180200186A1Efficient uptakeEfficient reductionPowder deliveryOrganic active ingredientsDiseaseSterol
Compositions comprising an RNA interference (RNAi) compound complexed to or encapsulated by lipid particles are provided. The lipid particle is a lipid nanoparticle, a liposome or a combination thereof. The lipid particle comprises a cationic lipid, a phospholipid, a sterol or a tocopherol or a derivative thereof, and a conjugated lipid. The invention also provides methods for treating pulmonary diseases or disorders such as pulmonary fibrosis and sarcoidosis using the compositions comprising the RNAi-lipid particles of the invention. The methods comprise administering one or more of the RNAi compositions to the lungs of the patient in need thereof via an inhalation delivery device, for example, a nebulizer, dry powder inhaler, or a metered dose inhaler.
Owner:INSMED INC
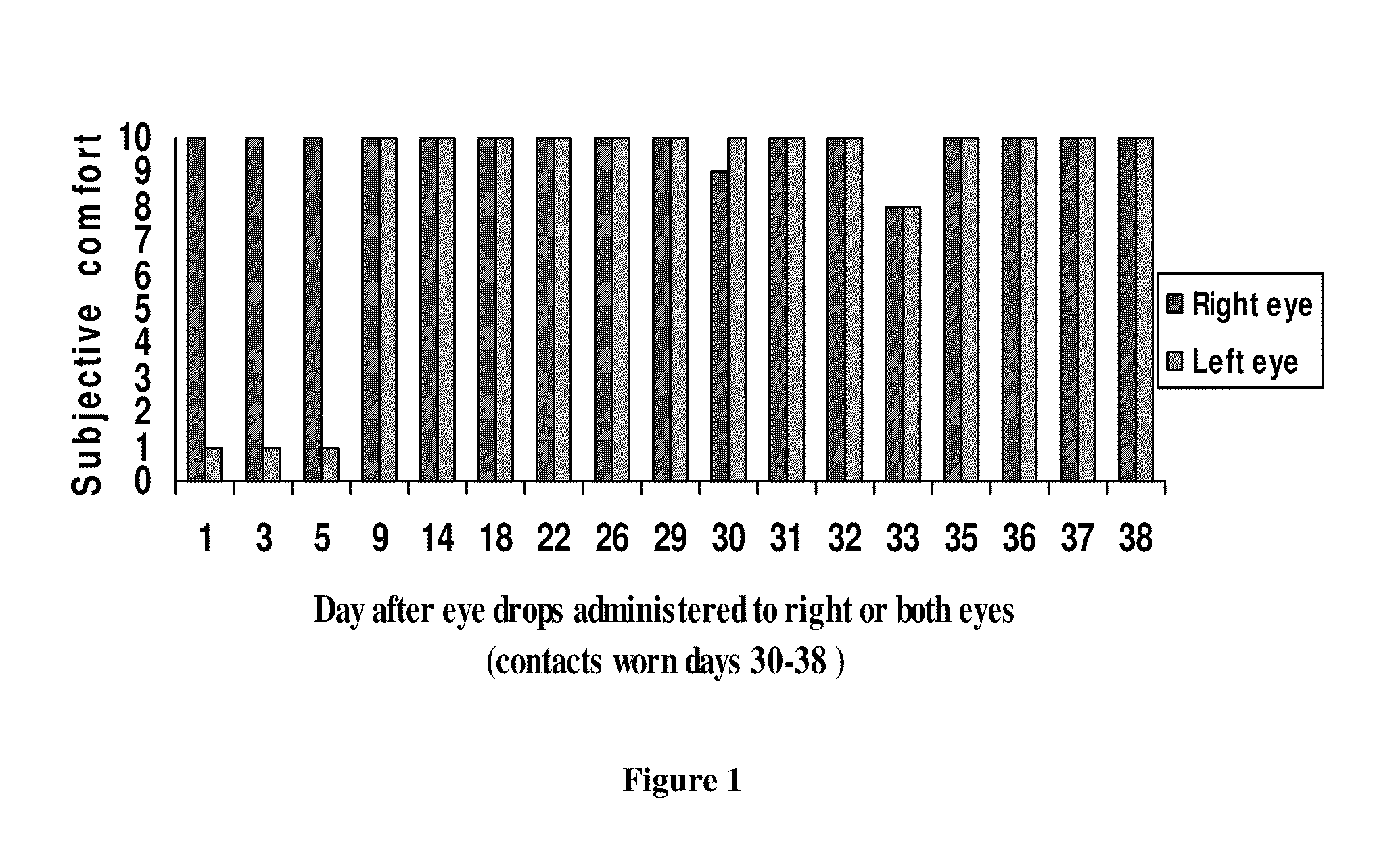
Composition and method for treating dry eye syndrome
ActiveUS8722728B2Safe, long-lasting, and relatively inexpensiveBiocideSenses disorderAcetic acidTocopheryl acetate
The present invention provides for compositions, medicaments, and methods for treating or alleviating the symptoms of dry eye syndrome or chronic dry eye. More specifically, the present embodiments provide for medicaments consisting of tocopherol or tocotrienol eyedrops. A single topical administration of tocopherol or tocotrienol eyedrops in the eyes of a subject suffering from dry eye alleviates symptoms for at least one day. In particular, the eyedrop medicament consists of α-tocopheryl acetate; α-tocopheryl acetate and about 0.5% aqueous component; or α-tocopheryl acetate, about 2.5% tocopherol emulsifier, and about 20% to about 30% aqueous excipient.
Owner:RAMSCOR
Nutritional compositions including rrr-alpha tocopherol and polyunsaturated fatty acids
InactiveUS20150025133A1Improved central nervous system maturationImproved cognitive developmentBiocideSugar food ingredientsBrain developmentIodo fatty acid
Disclosed are nutritional formulas generally, and infant formulas specifically, including a combination of RRR-alpha tocopherol, LC-PUFAs, and optionally vitamin C. The combination enhances brain development and improves cognitive performance in an individual, and specifically in an infant.
Owner:ABBOTT LAB INC
Composition and method for treating dry eye syndrome
ActiveUS20100093845A1Safe, long-lasting, and relatively inexpensiveBiocideSenses disorderAcetic acidExcipient
The present invention provides for compositions, medicaments, and methods for treating or alleviating the symptoms of dry eye syndrome or chronic dry eye. More specifically, the present embodiments provide for medicaments consisting of tocopherol or tocotrienol eyedrops. A single topical administration of tocopherol or tocotrienol eyedrops in the eyes of a subject suffering from dry eye alleviates symptoms for at least one day. In particular, the eyedrop medicament consists of α-tocopheryl acetate; α-tocopheryl acetate and about 0.5% aqueous component; or α-tocopheryl acetate, about 2.5% tocopherol emulsifier, and about 20% to about 30% aqueous excipient.
Owner:RAMSCOR
Oral Care Compositions Containing a Mixed Tocopherol Component
InactiveUS20080241117A1Enhance systemic healthGood for healthAntibacterial agentsOrganic active ingredientsDelta-TocopherolMedicine
An oral care composition, such as a dentifrice composition, which provides enhanced anti-gingivitis efficacy is disclosed. The composition includes a tocopherol component which consists of about 10% to about 90% of gamrnma tocopherol, with the balance of the components selected from alpha tocopherol, beta tocopherol, delta tocopherol, and mixtures thereof.
Owner:COLGATE PALMOLIVE CO
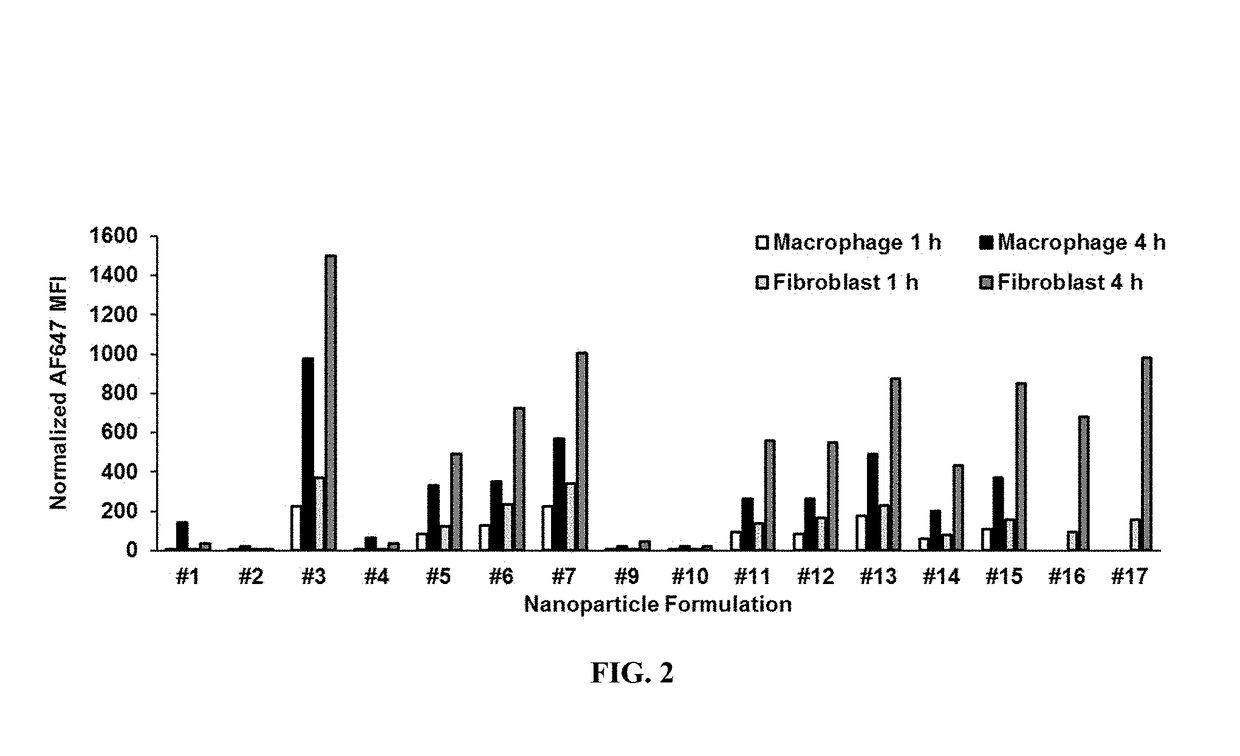
Environmental response type anti-tumor nanometer medicine with high medicine loading capacity, carrier and preparation method thereof
InactiveCN108066771AHigh embedding rateGood biocompatibilityOrganic active ingredientsNanomedicineFiberPolyethylene glycol
The invention relates to an environmental response type anti-tumor nanometer medicine with high medicine loading capacity, a carrier and a preparation method thereof. The nanometer medicine comprisesan amphiphilic polymer carrier and a medicine prodrug; the amphiphilic polymer carrier comprises a hydrophilic segment and a hydrophobic segment which are connected mutually; the hydrophilic segment comprises polyethylene glycol or poly(N-(2-hydroxypropyl)methacrylamide); the hydrophobic segment comprises D-alpha-tocopherol succinate; the medicine prodrug comprises a medicine for treating tumor aswell as a hydrophobic chain segment which is connected with the medicine through a chemical bond; and the hydrophobic chain segment comprises D-alpha-tocopherol succinate. The nanometer medicine hasthe characteristics of high medicine loading capacity, environmental responsiveness and adjustable and controllable grain size, and can achieve the properties that the tumor penetration ability is high, the tumor cell multidrug resistance is achieved, drug-resistant cells are killed in advance, tumor related fibroblast is not killed and tumor stem cells can be killed effectively according to composition of different polymers and the loaded prodrug molecule types.
Owner:北京思如诺科技有限公司
Antioxidant Formulations
InactiveUS20130251865A1Maintain solubilityGood effectMilk preservationFruit and vegetables preservationGreen tea extractBULK ACTIVE INGREDIENT
Antioxidant formulations containing new active molecules with tocopherols are disclosed. The best performing formulas contain extracts of green tea that are oil soluble, extracts of rosemary, extracts of spearmint and tocotrienols. Interestingly, the amount of tocopherols in formulas could be reduced by 50% in this diet when the other actives were increased accordingly.
Owner:BANK OF AMERICA N A
Mitochondrially Delivered Anti-Cancer Compounds
ActiveUS20110105437A1Induced deathSuitable isotonicityBiocideOrganic active ingredientsCancer cellMedicine
This invention relates to anti-cancer compounds and to methods for treating or preventing cancer. In one aspect the invention concerns mitochondrially delivered pro-oxidant anti-cancer compounds that generate reactive oxygen species and induce apoptosis of cancerous cells. The delivery moiety can be a lipophilic cation and the pro-oxidant vitamin E analogue, such as α-tocopheryl succinate, α-tocopheryl maleate, α-tocopheryl maleyl amide, or 2,5,7,8-tetramethyl-2R-(4R,8R,12-trimethyltridecyl)-chroman-6-yloxyacetic acid (α-tocopheryloxyacetic acid).
Owner:CANCURE LTD
Sprayable pharmaceutical compositions for topical application comprising sucralfate gel
InactiveUS20130274209A1Improved spray patternSuperior dermal adhesionBiocideCarbohydrate active ingredientsSecond-Degree BurnSucrose sulfate
A pharmaceutical composition is described comprising sucralfate gel, PVA and optionally a vegetable product comprising tocotrienols and tocopherols. In particular, this composition proved to be suitable for being sprayed, and is advantageously used in the treatment of skin lesions, even deep lesions, such as second-degree burns.
Owner:LAB ITAL BIOCHIM FARM LISAPHARMA
Compositions and method for the reduction of post-operative pain
ActiveUS8603528B2Flexibility and variation availabilityExtended elution timePowder deliveryPharmaceutical non-active ingredientsCarboxylic acidKetone
At least three component, body-implantable, absorbable, biocompatible, putty, and non-putty pain-relieving compositions for use in surgery comprising in intimate admixture: an analgesic having local pain-relieving activity for internal relief of pain, a finely powdered bulking material, preferably less than 50 microns, e.g. the metal salts of fatty acid, hydroxyapatite, DBM, polyglycolide, polylactide, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches.An organic liquid capable of solubilizing, dispensing or suspending the analgesic, such as esters of monohydric alcohols with aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with mono or polycarboxylic acids; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; polyhydroxy compounds and esters and ethers thereof; random or block copolymers of ethylene oxide and propylene oxide.
Owner:ORTHOCON INC
Less harmful nontoxic rubber automobile seal strip
The invention provides a less-harmful nontoxic rubber vehicle sealing strip which has excellent processability and physical properties and belongs to an environment friendly sulfidizing system. Main raw materials are as follows: 200 plus or minus 10 portions of ethylene propylene diene methylene, and 150 plus or minus 10portions of carbon black; 20 plus or minus 2 portions of light super fine calcium carbonate and 30 plus or minus 3 portions of talc as fillers; 20 plus or minus 2 portions of high-flash paraffin oil or styrene / Alpha-methyl styrene / vinyl toluene terpolymer as a softener; 10 plus or minus 1 portions of aluminum hydroxide as a flame retardant; 5 plus or minus 0.5 portions of zinc oxide and 2 plus or minus 0.2 portions of stearic acid as active agents together; 4 plus or minus0.4 portions of polyethylene glycol of a hydroxyl polar group as an additive; 5 plus or minus 0.5 portions of calcium oxide as a neutralizer; 4 plus or minus 0.4 portions of nitrogen-free sulfidationmaterial which is mainly sulphur; and 0.3 plus or minus 0.03 portion of Alpha-tocopherol as an inhibitor. The less harmful nontoxic rubber vehicle sealing strip can not only reduce environmental pollution, but also maintain reasonable cost.
Owner:ANHUI CHENYANG RUBBER & PLASTRIC CO LTD
Mineral spring rejuvenating lotion and preparation method thereof
InactiveCN110151588AEasy to useTo promote metabolismCosmetic preparationsToilet preparationsCuticleSkin color
The invention relates to the field of skin care products, in particular to a mineral spring rejuvenating lotion and a preparation method thereof. The mineral spring rejuvenating lotion comprises water, 1,3-propylene glycol, glycerinum, trehalose, nicotinamide, cyanocobalamin, phenoxyethanol, allantoin, sodium hyaluronate, 3-o-ethyl ascorbic acid, panthenol and hydrolyzed collagen. Through a synergistic effect of multiple vitamins of the nicotinamide, 3-o-ethyl ascorbic acid, panthenol, cyanocobalamin and tocopherol, in cooperation with raw materials such as hydrolyzed collagen, trehalose and sodium hyaluronate, the mineral spring rejuvenating lotion is prepared, skin pores are purified, convenience is provided for the skin to absorb nutrient components, the water flocculation texture of the skin is restored, the translucent feeling and softness of the cuticle are maintained, and a mineral spring skin care lotion is supplemented, so that the skin feels active, moisturized and transparent; the vitamin B12 and sodium hyaluronate supplement each other, the skin metabolism is improved, absorption is promoted, the self-protection force of the substrate is effectively improved, and the conditions of dull skin and lowered elasticity are reduced; the spots are faded, the skin color is balanced, whiting and shining are achieved, the skin is transparent and free of speckles, and the skinis lustrous, transparent, bright and white.
Owner:广州维真渼生物科技有限公司
Water-soluble compositions of bioactive lipophilic compounds
Water-soluble compositions comprising a lipophilic compound and a solubilizing agent of the general formula:{X—OOC—[(CH2)n—COO]m}p—Y (I)wherein:X is a residue of a hydrophobic moiety,Y is a residue of a hydrophilic moiety,p is 1 or 2,m is 0 or 1, andn is an integer greater than or equal to 0are disclosed. The lipophilic compound is preferably selected from the group consisting of water-insoluble ubiquinones, ubiquinols, vitamins, provitamins, polyene macrolide antibiotics, and mixtures thereof. The hydrophobic moiety is preferably a sterol or a tocopherol and the hydrophilic moiety is preferably a polyalkylene glycol. In some embodiments, the sterol is cholesterol or sitosterol, the tocopherol is α-(+)-tocopherol, the polyalkylene glycol is a polyethylene glycol or its methyl monoether having an average molecular weight between 400 and 1000, p is equal to 1 or 2, m is equal to 0 or 1 and n is an integer between 2 and 18.
Owner:NAT RES COUNCIL OF CANADA
Stable antioxidant compositions
ActiveUS20180325792A1Surprising solubility and stabilityParticularly effective for treating and protecting the skinOrganic active ingredientsCosmetic preparationsSolubilityWater soluble
The present disclosure relates to antioxidant compositions comprising a high amount of stabilized neohesperidin dihydrochalcone. The antioxidant compositions may further include additional antioxidants, such as ferulic acid, and tocopherols. The compositions include at least 20 wt. % of one or more water-soluble solvents, such as propanediol, which contribute to the surprising solubility and stability of the compositions. The antioxidant properties of the compositions make them particularly effective for treating and protecting the skin. For example, the compositions are useful for protecting the skin from environmental insult.
Owner:LOREAL SA
Method of enhancing bioavailability of DHA and other lipid-soluble nutrients
A method of enhancing the bioavailability of a lipid-soluble nutrient, such as DHA, arachidonic acid, tocopherol, and carotenoids, in a newborn or preterm infant is disclosed. In this method, the lipid-soluble nutrient is administered in a nutritional product (such as an infant formula) to a preterm or newborn infant. The nutritional product comprises (a) an effective amount of the lipid-soluble nutrient; and (b) a fatty component comprising a combination of: (i) at least 6 g / l predigested fat, wherein the predigested fat includes at least one of: monoglycerides, free fatty acids, or a combination of monoglycerides and free fatty acids, and (ii) at least 1.44 g / l phospholipid. A method of enhancing cognition in newborn or preterm infants utilizing the nutritional product is also disclosed.
Owner:ABBOTT LAB INC
Whitening cream and preparation method thereof
InactiveCN108113899AImprove securityImprove recognitionCosmetic preparationsToilet preparationsMagnesium phosphateSide effect
The invention belongs to the field of cosmetics, and in particular relates to whitening cream and a preparation method thereof. The whitening cream is prepared from the following raw materials: an emulsifier, tocopherol (vitamin E), laurocapram, water, EDTA disodium, xanthan gum, propylene glycol, glycerinum, polyacrylamide, C13-14 isoparaffin, laurinol polyether-7, 3-o-ethyl ascorbic acid, a double-layered surfactant, magnesium phosphate coating ascorbic acid and a preservative. As the whitening cream prepared by the proper amount of raw materials is free of unclear components, the whiteningcream is good in safety, high in degree of recognition of consumers and free of side effects compared with common whitening cream products. The whitening cream is good in whitening effect, can enhancethe brightness of skin effectively if being used for a long time, alleviates the synthetic speed of melanin effectively, and also plays the roles of complementing water, moisturizing, protecting skinand whitening. The whitening cream is safe and non-irritant to human skin, and is suitable for various crowds.
Owner:广州市茗妍化妆品有限公司
Water soluble compositions for bioactive lipophilic compounds
A solubilizing agent of the formula €ƒ€ƒ€ƒ€ƒ€ƒ€ƒ€ƒ€ƒ X-OOC-(CH 2 ) n -COO-Y wherein X is a member selected from campesterol, sitosterol, ergosterol and stigmasterol; Y is a member selected from polyalcohol, polyether, polyanion, polycation, polyphosphoric acid, polyamine, polysaccharide, polyhydroxy compound and polylysine, and derivatives thereof; and n is an integer selected from 0 to 18. The solubilizing agent may be included in a water-soluble composition comprising a bioactive lipophilic compound and the solubilizing agent.
Owner:NAT RES COUNCIL OF CANADA
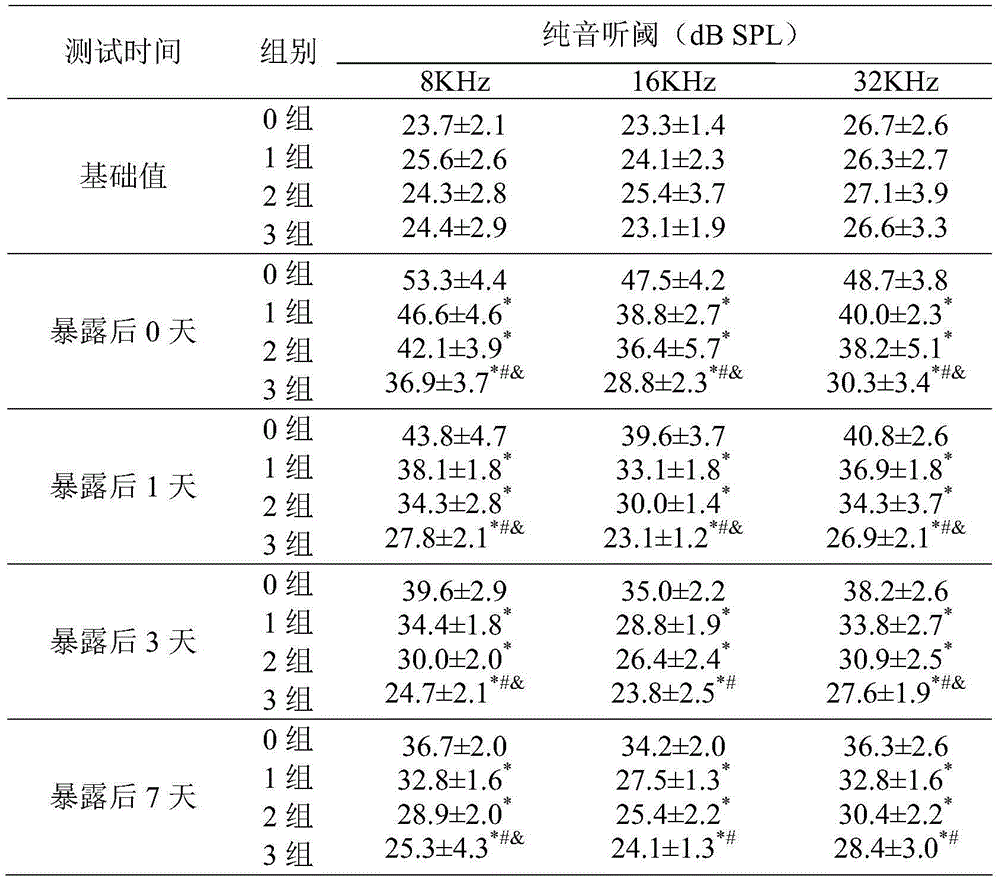
Nutritional agent with noise induced hearing loss resistance
ActiveCN104664407AGood noise immunity hearing lossPromote aerobic metabolismFood preparationPotential toxicityData display
The invention discloses a nutritional agent with noise reduced hearing loss resistance. The nutritional agent is prepared from the following raw materials in parts by weight: 600-1000 parts of N-acetyl-L-cysteine, 1-4 parts of vitamin A, 1-5 parts of vitamin B1, 1-5 parts of vitamin B2, 1-10 parts of vitamin B6, 0.01-0.05 part of vitamin B12, 60-1000 parts of vitamin C, 15-300 parts of vitamin E which is based on alpha-totaxin, 50-400 parts of magnesium and 79-3175 parts of food acceptable accessories. Experiment data displays that the nutritional agent has remarkable effect of preventing and controlling the noise induced hearing loss. The nutritional agent can be used for enhancing the anti-oxidation ability of the organism and lightening the exitotoxicity damage, has the characteristics of being remarkable in curative effect, low in potential toxicity and low in cost, and is suitable for the noise reduced hearing loss protection and daily nutrient supplementation of people who live and work in the environment with continuous exposed high-intensity noise.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
Formulations of hydrophilic compounds
A formulation includes a continuous hydrophobic phase, a hydrophilic phase, at least one hydrophilic compound substantially dissolved in the hydrophilic phase, and a stabilizing component. The stabilizing component can contain D-a-tocopheryl polyethylene glycol 1000 succinate (TPGS) and / or at least one emulsifier having a hydrophilic-lipophilic balance (HLB) value of at least 10. In one alternative, the stabilizing component contains a first, polysorbate emulsifier having an HLB value of at least 10, and a second emulsifier having an HLB value of less than 10.
Owner:BCS BUSINESS CONSULTING SERVICES PTE
All-in-one skin-brightening formulations
ActiveUS20170312215A1Improve skin textureLittle or no shelf lifeCosmetic preparationsToilet preparationsNarrow rangeKojic acid
Certain dermatological formulations for direct skin application that correct for hyperpigmentation effects are provided. Such formulations include a number of different compounds that work synergistically to effectuate multiple and varied pathways to pigment removal and / or brightening within the dermal layer. The ability to do so without the necessity for hydroquinone, and thus with a suitable composition that is safe for handling and utilization by pregnant and nursing women, is accorded through a combination of specific ingredients in limited proportional ranges, as well. Such ingredients include kojic acid, niacinamide, sodium hyaluraonate, tocopherol, and licorice extract in very narrow ranges proportionally within an aqueous serum. The method of manufacture and use thereof such formulations are encompassed within the invention as well.
Owner:PATEL PURVISHA
Tocopherol stabilisers for nitrocellulose-based propellants
ActiveUS20180029952A1Improve explosive powerGood curative effectNon-explosive stabilisersOrganic chemistryArylNitrocellulose
Owner:PB CLERMONT
Extraction method of tocopherol intermediate
InactiveCN102633766APrevent oxidative deteriorationIncrease contentOrganic chemistrySterol esterFatty acid methyl ester
The invention relates to an extraction method of a tocopherol intermediate, belonging to the technical field of organic complexing extraction. The tocopherol intermediate is a product obtained after aminomethylation reaction of non-D-alpha-tocopherol in a tocopherol concentrated solution. The tocopherol concentrated solution contains higher-content mixed tocopherol (D-alpha, beta, gamma and delta-tocopherol), also contains a great amount of glyceride, fatty acid methyl ester, sterol ester and other impurities, and the preparation of high-content D-alpha-tocopherol is hindered by the existence of the impurities. The boiling points of the impurities are close to that of tocopherol, therefore, the impurities cannot be separated from tocopherol by distilling and other methods. The non-D-alpha-tocopherol intermediate is a tertiary amine substance, has certain alkalinity, and can be combined with an acid to form a salt, and glyceride and other impurities are easy to dissolve in an organic solvent. According to the invention, the tocopherol intermediate is extracted from glyceride and other impurities by a liquid-liquid extraction method, and the obtained non-D-alpha-tocopherol intermediate is almost pure, thus the purpose of purification is achieved.
Owner:JIANGNAN UNIV +1
Method of preventing flavor component from degradation
InactiveUS7939120B2Appearance be impairedTaste be impairedMilk preservationFruit and vegetables preservationFlavorAlcohol
The present invention relates to a deterioration preventive for a flavor component, which is an oil-in-water and / or -polyhydric alcohol type emulsion, comprising an extracted tocopherol, wherein d-δ-tocopherol is contained in an amount of 45% by weight or more of a total tocopherol, ferulic acid and / or a derivative thereof, and a polyglycerol fatty acid ester; a deterioration preventive for a flavor component, which is an oil-in-water and / or -polyhydric alcohol type emulsion, comprising (A) the above-mentioned extracted tocopherol, (B) ferulic acid and / or a derivative thereof, and (C) an emulsifying agent having an HLB of 9 or more; a flavor for foodstuff, comprising the above-mentioned deterioration preventive; an emulsion flavor for foodstuff, comprising the above-mentioned extracted tocopherol, a catechin; and a polyglycerol fatty acid ester; and foodstuff comprising the above-mentioned deterioration preventive for a flavor component, the above-mentioned flavor for foodstuff, or the above-mentioned emulsion flavor for foodstuff.
Owner:TAIYO KAGAKU CO LTD
Preparation method of d-alpha-tocopherol polyethylene glycol succinate
ActiveCN108676157AEliminate the purification processOmit separabilityPalladium on carbonTocopherol succinate
The invention discloses a preparation method of d-alpha-tocopherol polyethylene glycol succinate. The preparation method comprises the following steps: by taking Merrifield resin and PEG1,000 as raw materials and alkali as a catalyst, performing reaction at 45-135 DEG C in an organic solvent to obtain resin loaded with PEG1,000; under the action of the catalyst, enabling the resin loaded with PEG1,000 and d-alpha-tocopherol succinate to be subjected to water diversion reflux reaction to obtain resin loaded with TPGS; and soaking the resin loaded with TPGS in toluene, then adding ethanol, palladium on carbon and phosphopyridoxal, and performing stirring reaction at 100-110 DEG C for 24 hours in hydrogen gas of 0.8-1.5MPa to obtain the d-alpha-tocopherol polyethylene glycol succinate. By adopting the synthetic method disclosed by the invention, the yield of TPGS is as high as 99%, the monoester content of TPGS is 99%, and the diester content of TPGS is 0.
Owner:NINGBO WANGLONG TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com