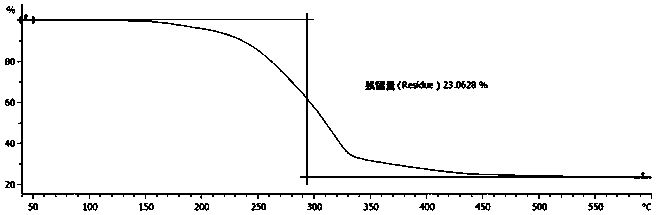
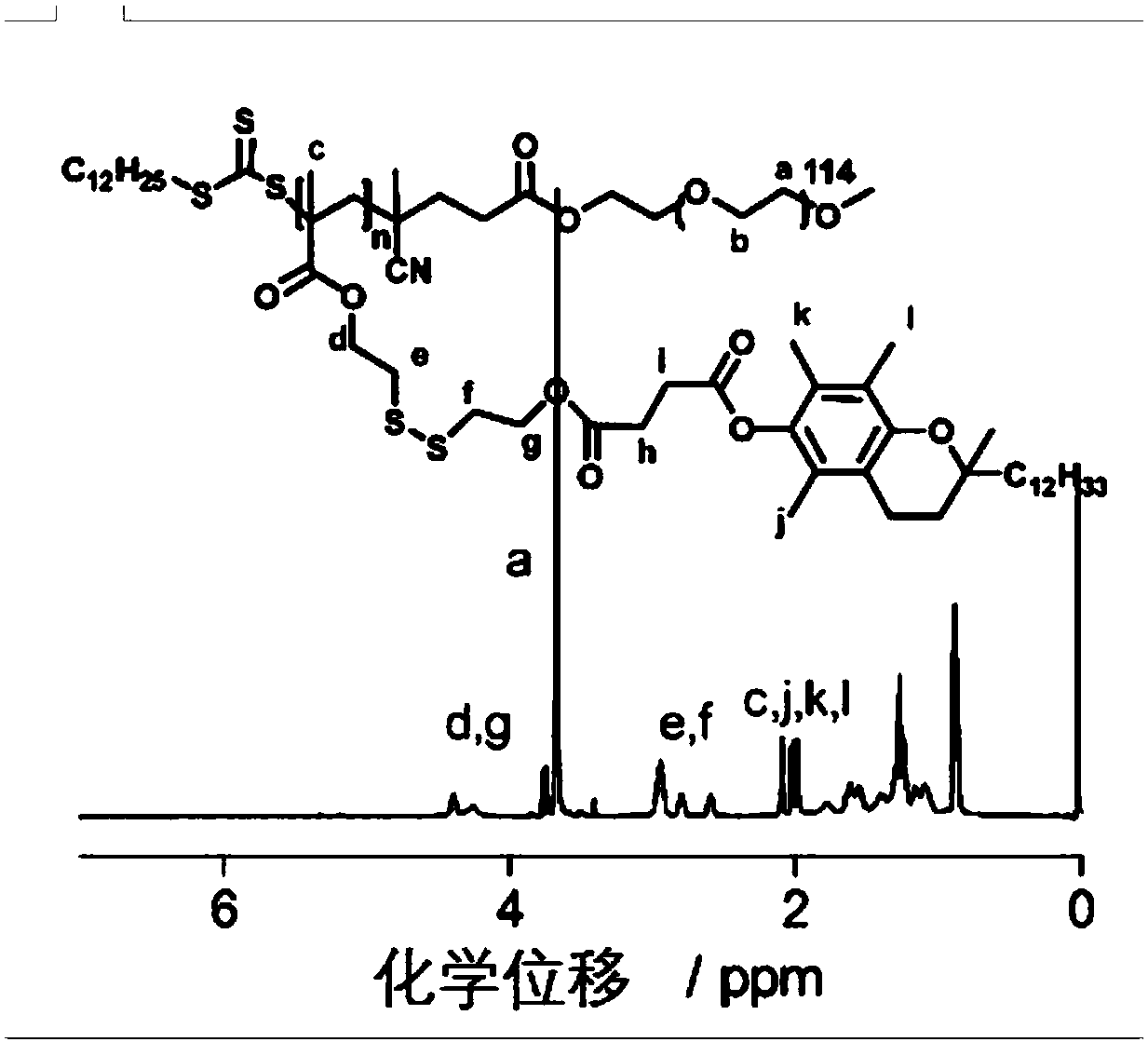
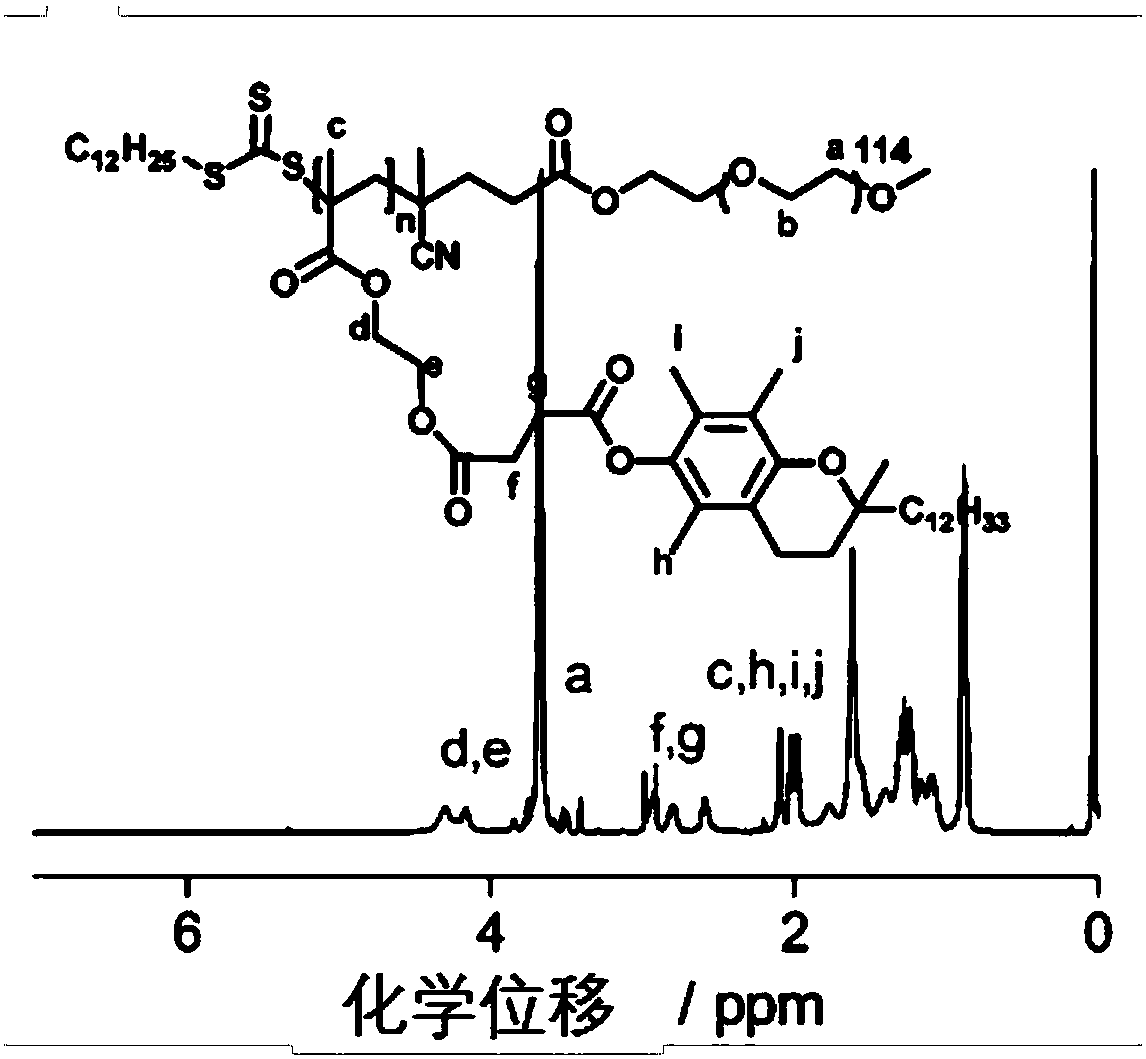
Environmental response type anti-tumor nanometer medicine with high medicine loading capacity, carrier and preparation method thereof
An environment-responsive, nano-medicine technology, applied in the fields of medicine and chemistry, can solve the problem of low content of terminal functional groups
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] PEG 5k -b-PMASSVE 5K synthesis method
[0083]
[0084] (1) 10 g of hydroxyl and methoxy terminated PEG with a number average molecular weight of 5000 were dissolved in 40 mL of toluene, and after azeotropic water removal twice with toluene, 4-dodecyl trithiocarbonate group- 3.23g of 4-cyanovaleric acid, 0.1g of 4-dimethylaminopyridine (DMAP) and 70ml of toluene, after complete dissolution, add 1.98g of N,N-dicyclohexylurea (DCC), and react in the dark for 96 hours at room temperature . After the reaction, dicyclohexyl urea (DCU) was removed by suction filtration, and then the filtrate was poured into excess ether, and the precipitate was collected. The resulting precipitate was dissolved in a small amount of toluene, and then precipitated with ether. Repeat this 3-5 times until the ether was colorless. The precipitate was vacuum-dried at 40° C. for 24 hours, and the obtained product was a macromolecular chain transfer agent of PEG.
[0085] (2) Under ice-water b...
Embodiment 2
[0088] Example 2 PEG 5K -b-PMACCVE 6K Synthesis
[0089]
[0090] (1) The synthetic method of PEG macromolecular chain transfer agent is the same as that of Example 1 (1).
[0091] (2) 0.5g of hydroxyethyl methacrylate (HEMA), 3.06g of VES, 0.95g of DCC, 0.094g of DMAP and 20ml of DCM were added to a 100ml round bottom flask, and stirred at room temperature overnight. DCU was removed by suction filtration, and purified by silica gel column chromatography using dichloromethane as the mobile phase to obtain the product MACCVE with a yield of 66.9%.
[0092] (3) Take 0.23g of PEG macromolecular chain transfer agent, 0.5g of MACCVE, 2mg of 4,4'-azobis(4-cyanovaleric acid) (V501), and 1ml of DMSO in a vacuum reaction tube, under liquid nitrogen freezing Vacuumize, then return to room temperature, fill with argon, repeat this process of freezing-thawing-filling with argon three times, and finally place the reaction tube in a 70°C oil bath for 48 hours under the protection of a...
Embodiment 3
[0093] Example 3 PHPMA 10K -b-PMASSVE 6K Synthesis
[0094]
[0095] (1) Take 20 mg of dodecyl trithiocarbonate, 0.6 g of N-2-hydroxypropyl methacrylamide (HPMA), and 2 mg of V501 dissolved in 2 ml of DMSO, freeze and pump three times, and react at 70 degrees Celsius for 24 hours. After the reaction is completed, pure PHPMA is obtained by acetone precipitation 10k . The reaction yield was 80%.
[0096] (2) Remove 0.3g PHPMA 10k , 1mg V501, 0.2gMASSVE were dissolved in 1ml DMSO, frozen and pumped three times and reacted at 70 degrees Celsius for 24 hours. After the reaction is completed, acetone precipitates to obtain pure PHPMA 10k -b-PMASSVE 5k . The reaction yield was 78%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com