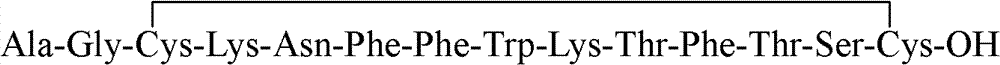Method for preparing somatostatin through solid-phase peptide synthesis
A technology for solid-phase peptide synthesis and somatostatin, which is applied to peptide preparation methods, chemical instruments and methods, peptides, etc., can solve the troublesome operation of pentapeptides, limit the large-scale production and use of somatostatin, and troublesome operation of dipeptides And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
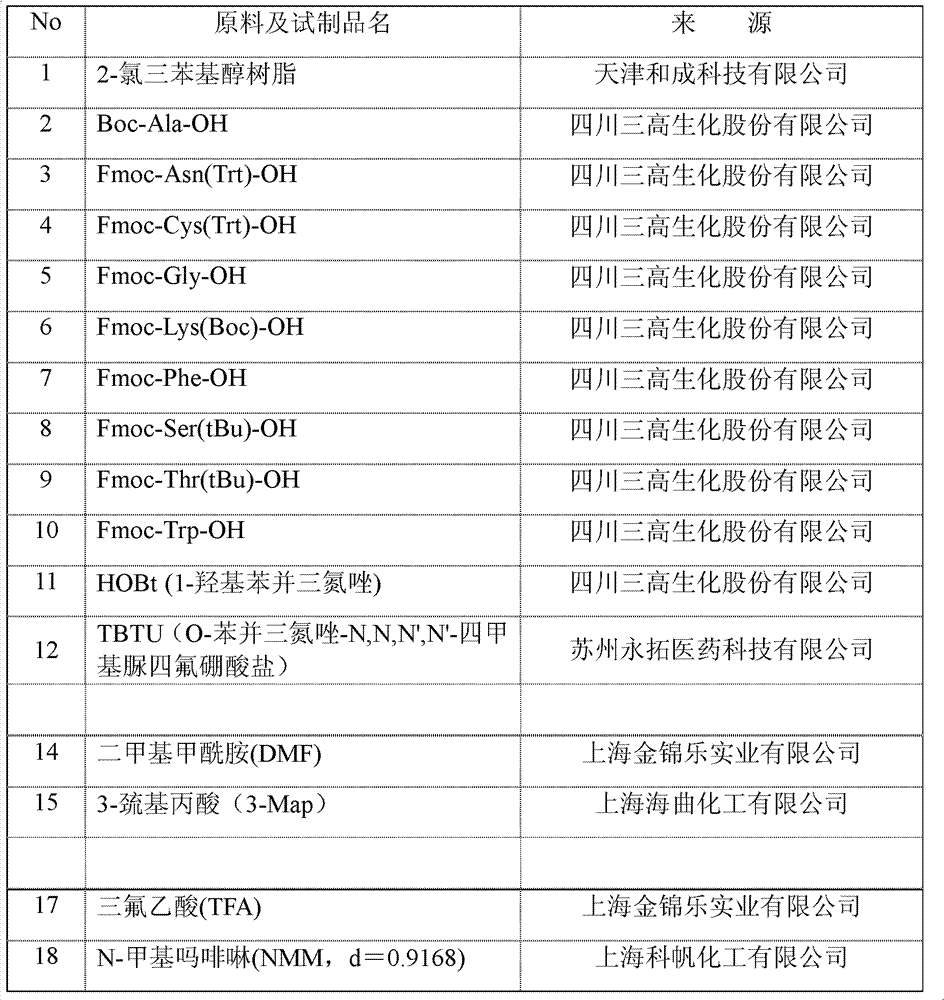
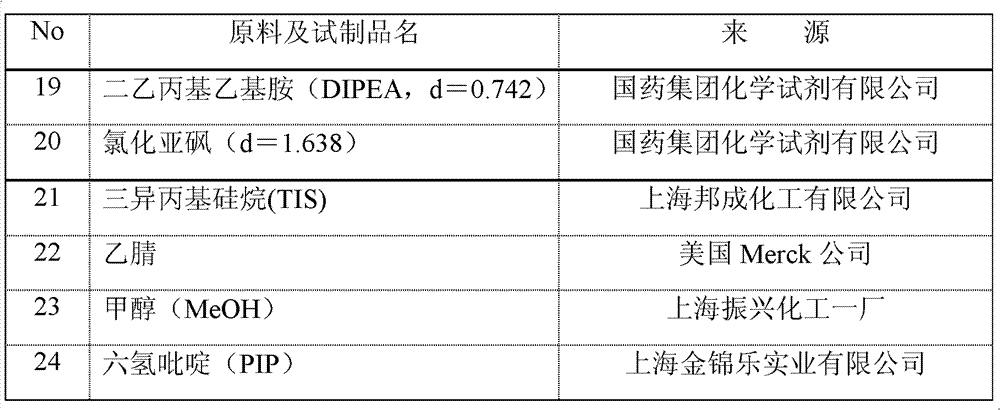
[0084] The list of raw materials adopted in the embodiment and the foregoing process is as follows:
[0085]
[0086]
[0087] Example 1
[0088] (1) Production of Fmoc-Cys(Trt)-resin
[0089] Weigh 2.22kg of 2-chlorotriphenyl alcohol resin (100-150 mesh, 0.7mmol / g), put it into a 50L polypeptide synthesizer, add 14LDCM, stir and swell at room temperature for 1 hour. Under stirring, 3.6 kg of thionyl chloride (d=1.638) was slowly added dropwise through a constant pressure funnel within 30 minutes, and then stirred and reacted at room temperature for 3 hours.
[0090] Drain, wash with 72LDCM 6 times, each time with 12LDCM, stir for 15 minutes and then drain.
[0091] Add 7L of DMF, then add 1.76kg of Fmoc-Cys(Trt)-OH dissolved in 7L of DMF, and 1.187kg of DIPEA, and react at 35±3°C for 1.5 hours. Add 2.5 L of methanol and react at 34.5±2.5°C for 30 minutes. Drained, the resin was washed once with methanol, washed five times with DMF, and dried.
[0092] (2) Productio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com