Pharmaceutical preparation containing exenatide
The technology of a pharmaceutical preparation and exenatide is applied in the field of stable pharmaceutical preparations of exenatide, and can solve the problems of affecting the quality of the medicine, affecting the curative effect, and difficult to ensure low-temperature storage conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
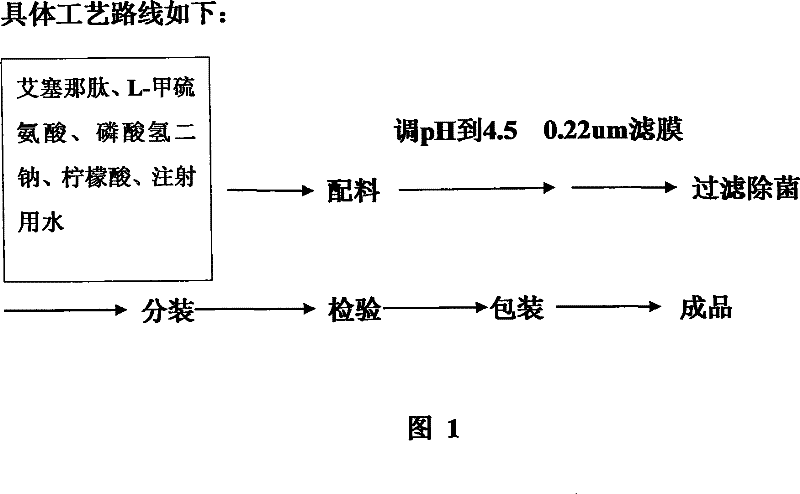
[0072] Preparation of Injectable Pharmaceutical Preparations Containing Exenatide
[0073] Take a certain amount of exenatide bulk drug, and calculate the final volume (V1) of the preparation when diluting it to 50 μg / ml based on the weight of the existing exenatide. According to V1, an appropriate amount of methionine was weighed and added to an appropriate amount of sterile water to dissolve, so that the content of methionine in the final preparation was 3% (W / V). Then add an appropriate amount of high-concentration sodium hydrogen phosphate-citrate buffer solution with a pH of 4.5, so that the final concentration of the buffer solution in the preparation is 20 mmol / L. Then put the weighed exenatide bulk drug into dissolution, and adjust the pH to 4.5 with 1mol / l HCL or 10% NaOH. Finally, add an appropriate amount of sterile water for dilution to make the final volume of the preparation reach V1, and use a 0.22 micron filter membrane to carry out sterile filtration of the p...
example 2
[0075] Stability comparison of different formulations of water for injection containing exenatide.
[0076] 1) Preparation prescription 1 (refer to BYTTA, the preparation product of exenatide that has been developed and marketed according to US patents (US6902744 and US7115569) preparation prescription)
[0077] Weigh 40g of mannitol and 2.2g of m-cresol and add them into 700ml of sterile water to dissolve, add 150ml of 0.2M acetic acid-sodium acetate buffer solution with a pH of 4.5, add the weighed 50mg of exenatide raw material into the solution, and use 1mol / l HCL or 10% NaOH to adjust the pH to 4.5. Finally, an appropriate amount of sterile water was added for dilution to make the final volume of the preparation reach 1000 ml, and the final concentration of exenatide was 50 μg / ml. The formulation was sterile filtered using a 0.22 micron filter. Fill in 0.2ml / bottle into 1ml prefilled needles, and the batch number is batch 01.
[0078] 1000 ml of a preparation with a ...
example 3
[0090] Screening Experiment of Exenatide Injection Storage Containers
[0091] Fill exenatide injection with 0.2ml / bottle into 1ml prefilled needles, vials and ampoules, respectively, and place them at -20°C and 4°C for investigation for two weeks, and take samples every week once. The two indicators, the adsorption of exenatide injection by the container and the utilization rate of the liquid by the container, were investigated.
[0092] 1. The adsorption effect of the container on exenatide injection: use RP-HPLC to detect the peptide content, and calculate the adsorption rate of the container on exenatide injection.
[0093] 2. The usage rate of the container for the liquid medicine: take 20 exenatide injections in different containers, weigh their total weight (W1) first, and then weigh their total weight after the liquid medicine is dispensed (W2), the total weight of the medicine that can be used (W3=W1-W2), the weight of 20×0.2ml exenatide injection is W4, then the us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com