Hydrochloric clindamycin nano granule formulation for injections and preparation thereof
A technology of clindamycin hydrochloride and nanoparticles, which is applied in the field of medicine, can solve problems such as poor stability of clindamycin hydrochloride, unsafe clinical medication, and inconspicuous effect, and achieve rapid and simple reaction process, low cost and high product quality. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
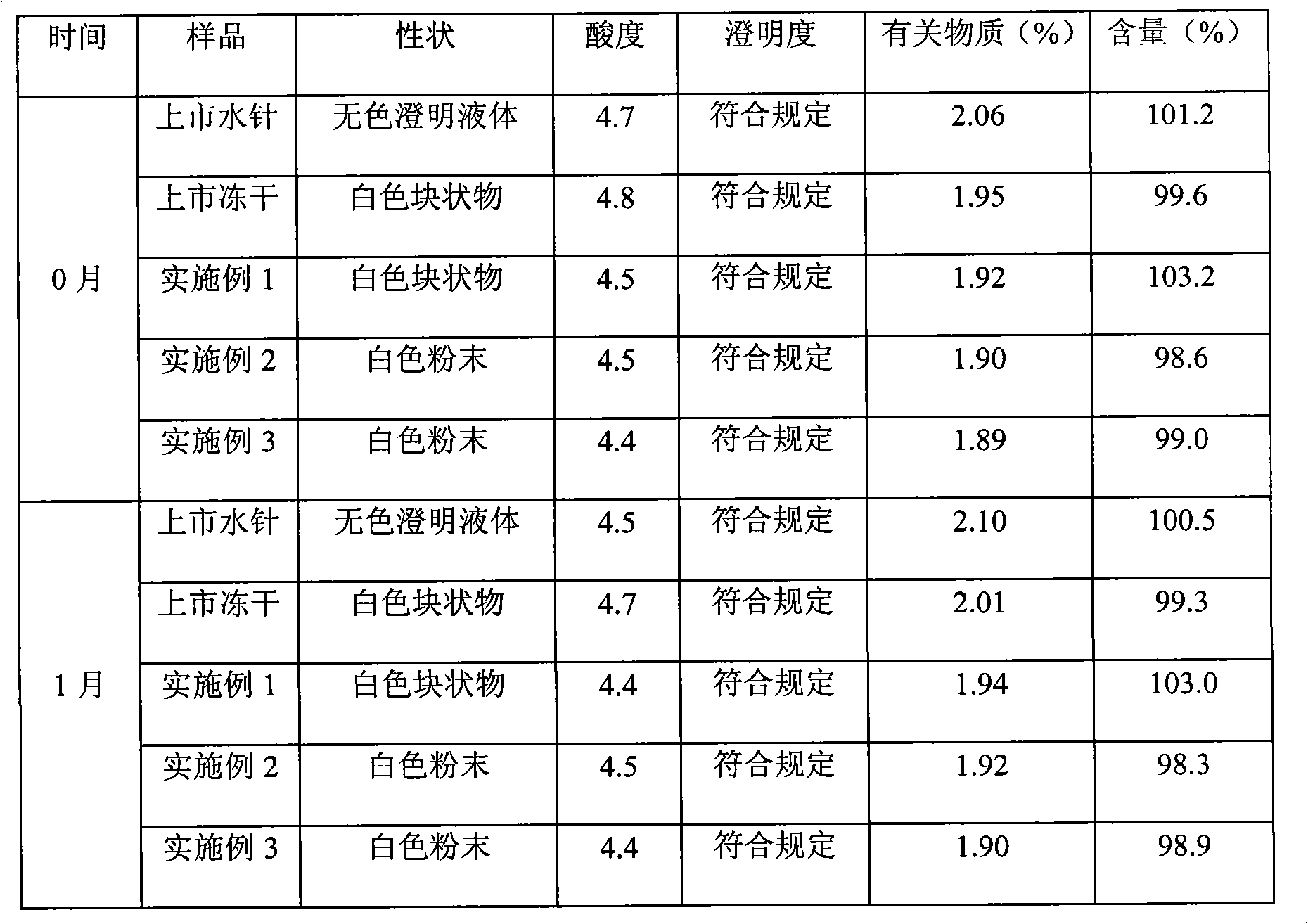
Embodiment 1
[0026] prescription:
[0027] Clindamycin Hydrochloride 75g
[0028] β-cyclodextrin 5g
[0029] poloxamer188 5g
[0031] Propyl cyanoacrylate 150g
[0032] Mannitol 10g
[0033] 1. Preparation process
[0034] (1) Weigh 75g of clindamycin hydrochloride, 5g of β-cyclodextrin and 5g of poloxamer188, add 500ml of water for injection to dissolve, adjust the pH value to 2.1 with 1mol / L hydrochloric acid solution, and stir evenly;
[0035] (2) Slowly add 150g propyl cyanoacrylate under electromagnetic stirring, continue stirring at room temperature for 3 hours, add 25g sodium sulfate, continue stirring for 3 hours, adjust the pH value to 4.9 with 1mol / L sodium hydroxide solution, and stir evenly ;
[0036] (3) Add 10g of mannitol, stir to dissolve, then add needle-use charcoal with 0.1g needle-use charcoal according to 100ml solution, stir at room temperature for 10 minutes, filter for decarburization, and then use 0.22μm microporous membrane filte...
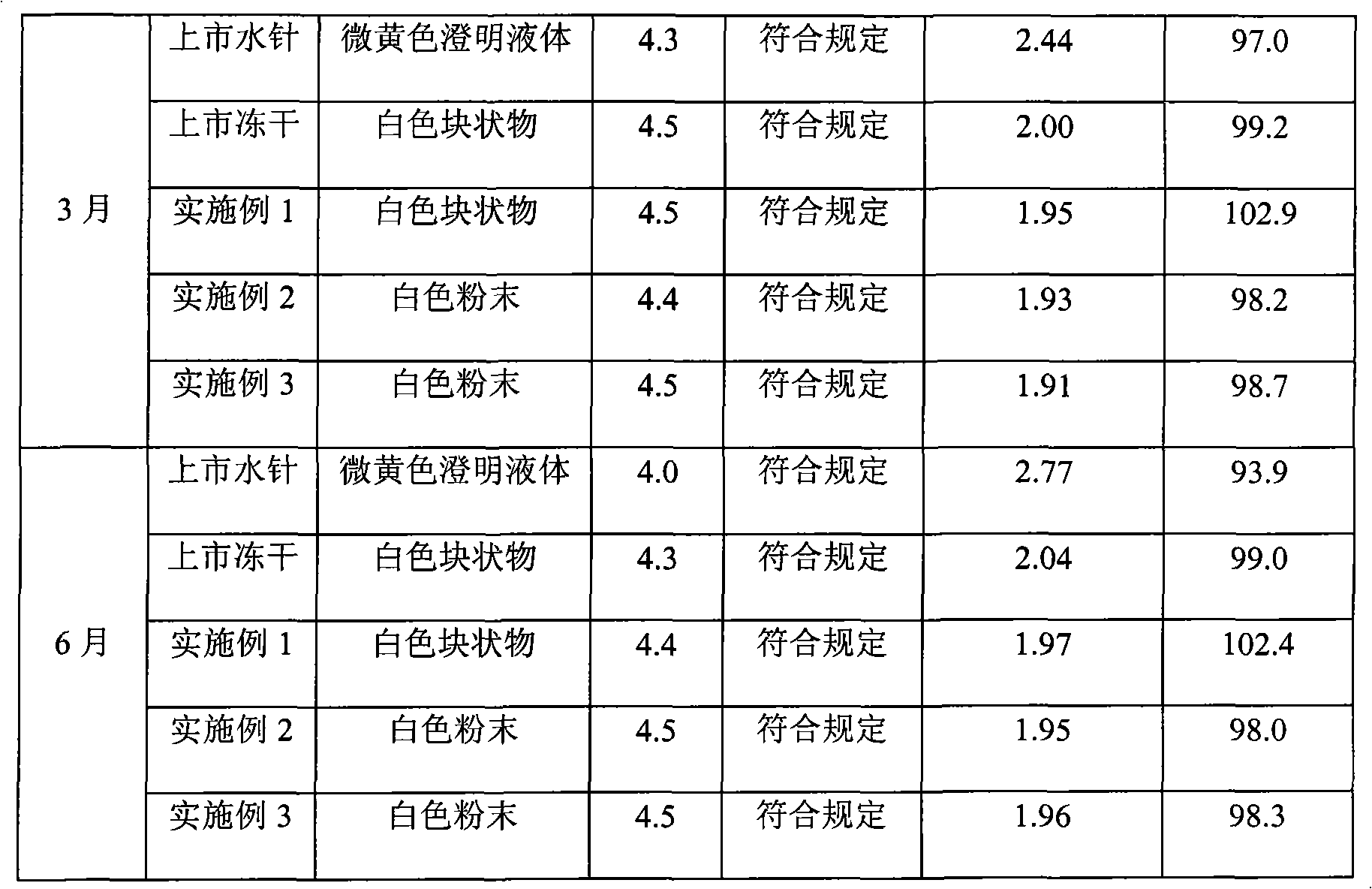
Embodiment 2
[0045] prescription:
[0046] Clindamycin Hydrochloride 300g
[0047] Dextran 80g
[0048] Povidone K30 80g
[0049] Sodium sulfate 200g
[0050] Methyl cyanoacrylate 800g
[0051] Glucose 400g
[0052] 1. Preparation process
[0053] (1) Take by weighing 300g of clindamycin hydrochloride, 80g of dextran and 80g of povidone K30, add 3000ml of water for injection to dissolve, adjust the pH value to 2.3 with a 1mol / L hydrochloric acid solution, and stir evenly;
[0054] (2) Slowly add 800g methyl cyanoacrylate under electromagnetic stirring, continue to stir at room temperature for 3 hours, add 200g of sodium sulfate, continue to stir for 3 hours, adjust the pH value to 4.8 with 1mol / L sodium hydroxide solution, and stir well ;
[0055] (3) Add 400 g of glucose, stir to dissolve, then add 0.3 g of needle charcoal to 100 ml of solution, stir at room temperature for 15 minutes, filter for decarburization, and then fine filter with a 0.22 μm microporous membrane to obtain gram...
Embodiment 3
[0060] prescription:
[0061] Clindamycin Hydrochloride 900g
[0062] Poloxamer 188 150g
[0063] Hydroxypropyl-β-cyclodextrin 150g
[0064] Sodium sulfate 450g
[0065] Ethyl cyanoacrylate 1800g
[0066] Lactose 1000g
[0067] 1. Preparation process
[0068] (1) Weigh 900g of clindamycin hydrochloride, 150g of Poloxamer188 and 150g of hydroxypropyl-β-cyclodextrin, add 6000ml of water for injection to dissolve, adjust the pH value to 2.5 with 1mol / L hydrochloric acid solution, and stir evenly;
[0069] (2) Slowly add 1800g of ethyl cyanoacrylate under electromagnetic stirring, continue to stir at room temperature for 3 hours, add 450g of sodium sulfate, continue to stir for 3 hours, adjust the pH value to 4.7 with 1mol / L sodium hydroxide solution, and stir evenly ;
[0070] (3) Add 1000 g of lactose, stir to dissolve, then add 0.5 g of charcoal for needles per 100 ml of solution, add charcoal for needles, stir at room temperature for 30 minutes, decarburize by filtratio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com