Adapalene and hydrochloric clindamycin compound gel preparation and preparation method thereof
A technology of clindamycin hydrochloride and compound gel, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of difficulty in obtaining hydrogel preparations and unsatisfactory treatment effects. , low stability of preparations, etc., to achieve broad application and market prospects, significant therapeutic effect, and convenient administration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
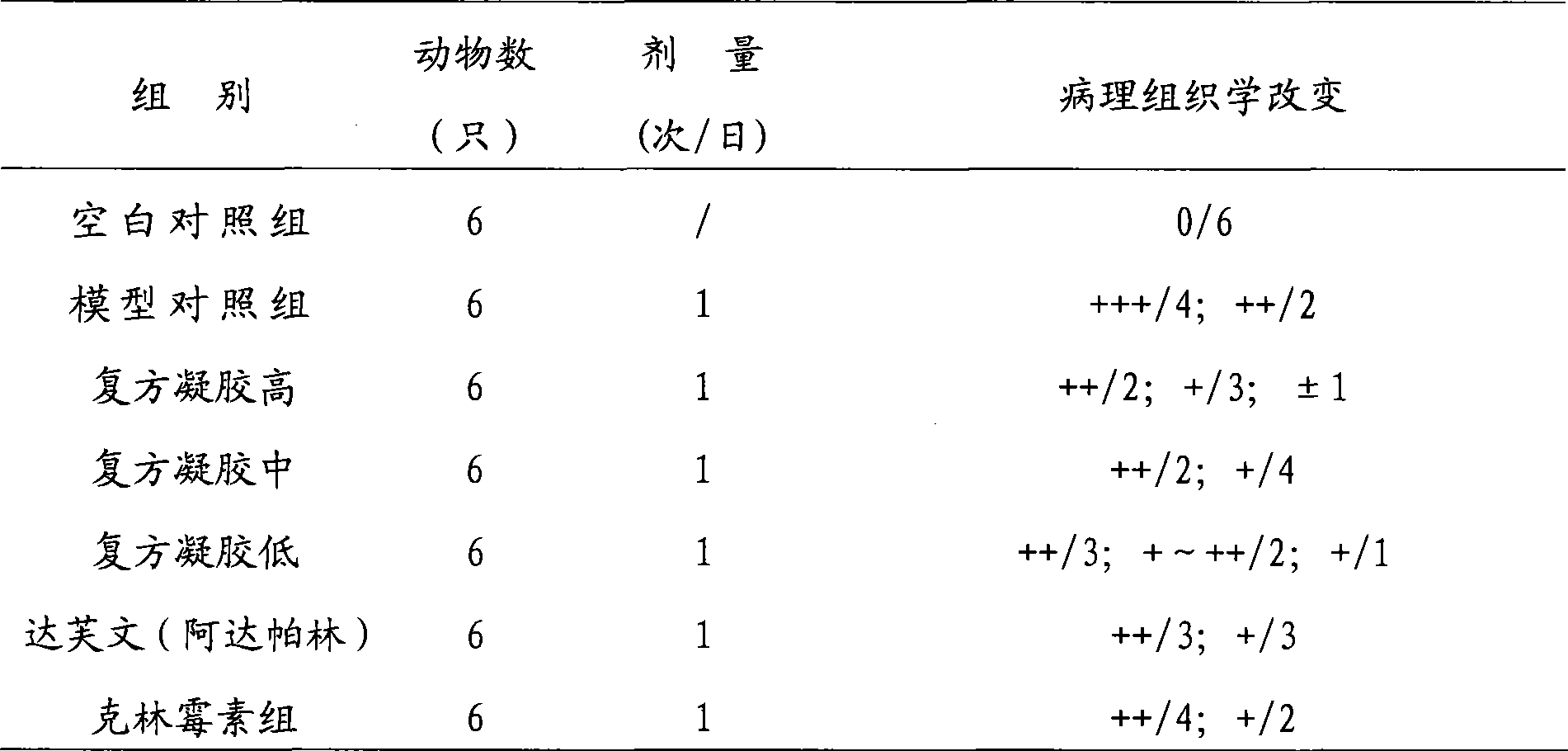
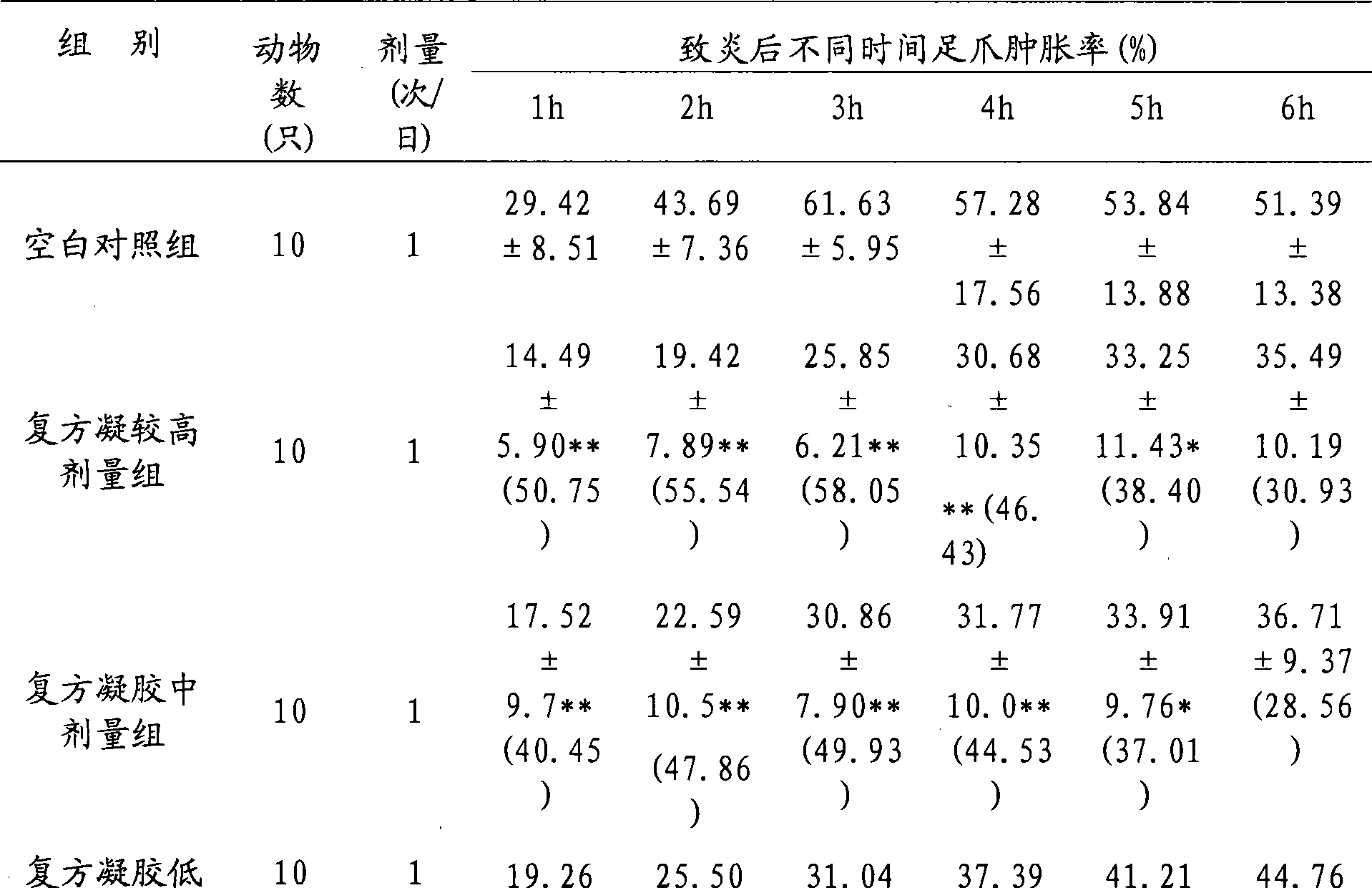
Examples
Embodiment 1
[0036] Embodiment 1 The preparation of compound gel of the present invention
[0037] Table 1 shows the amount of raw and auxiliary materials used in the preparation examples I-III of the compound gel preparation of the present invention.
[0038] Table 1 Raw materials and auxiliary material formulas used in preparation examples I-III
[0039] Element Preparation I
preparation Embodiment I
[0041] (1) prepare bulk drug and adjuvant by the preparation embodiment 1 in table 1;
[0042] (2) Preparation of gel matrix:
[0043] Take a 10L container, add 50g Carbomer 940, 5g disodium edetate, 10g methylparaben, 400g 1,2-propanediol, 16g poloxamer 188 into the container, add 2075.5g deionized water, stir until a uniform jelly-like matrix is formed. A 0.2 g / ml triethanolamine solution was prepared, and the remaining amount of 1,2-propanediol and 20 g of ethylene glycol phenyl ether were mixed. The jelly matrix, 0.2 g / ml triethanolamine solution, poloxamer 188 aqueous solution, 1,2-propanediol and ethylene glycol phenyl ether mixture, and deionized water were sterilized by heating at 121° C. for 20 minutes. Remove, cool, and set aside.
[0044] (3) Add bulk drug: dissolve 50g clindamycin hydrochloride with 375.1g deionized water under aseptic conditions, dissolve 5g adapalene with the above-mentioned sterilized good 1,2-propanediol and ethylene glycol phenyl ether mi...
Embodiment 2
[0046] Embodiment 2 The stability research of compound gel preparation of the present invention
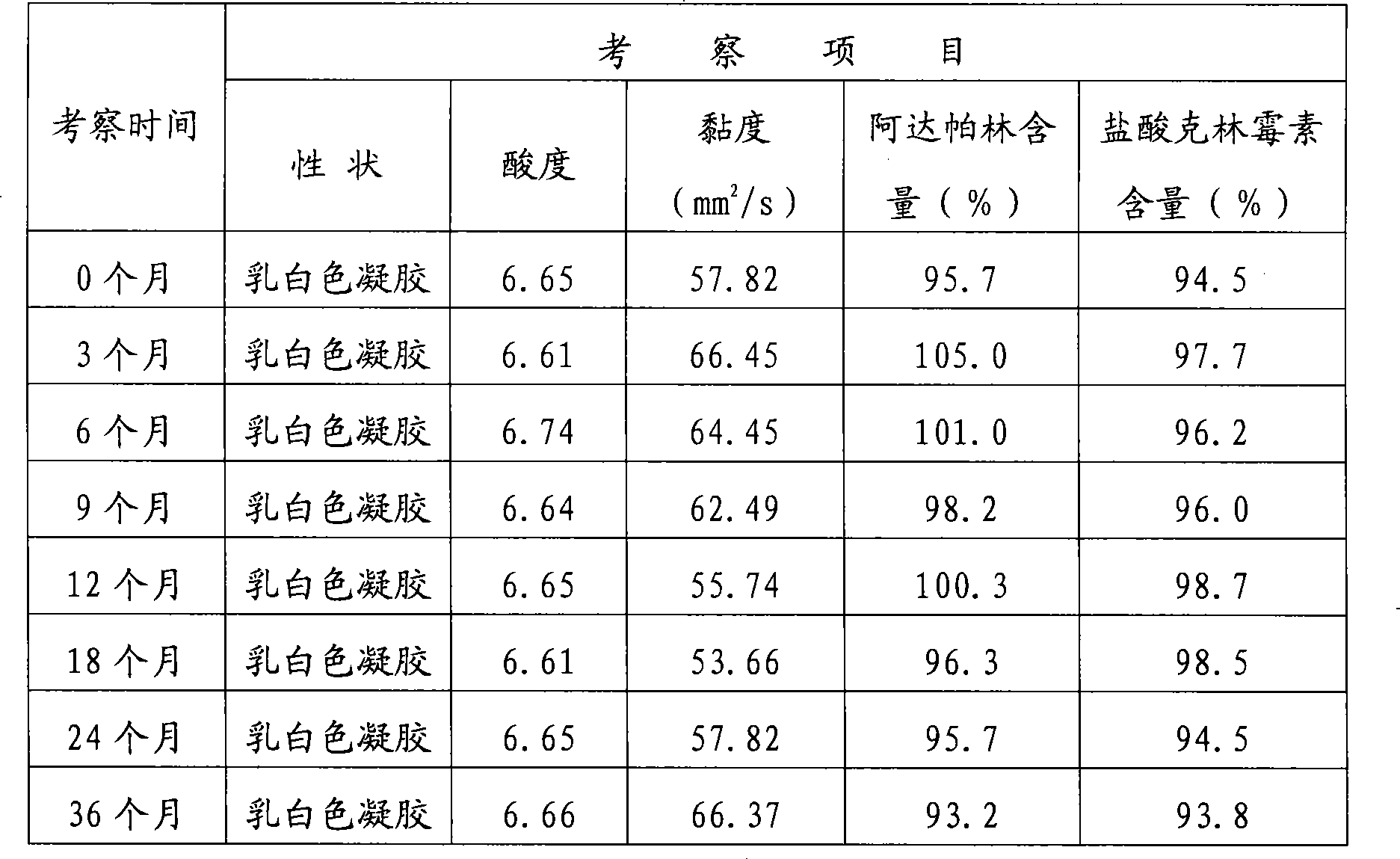
[0047] The stability investigation data of this product (batch number: 20040204) is shown in Table 2.
[0048] Table 2 The stability test results of adapalene hydrochloride clindamycin compound gel
[0049]
[0050] As can be seen from the 36-month stability test results of the compound preparation of the present invention, each quality index meets the standard requirements, and there is no obvious change. Stability study data show that the quality of the preparation is stable within the validity period.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com