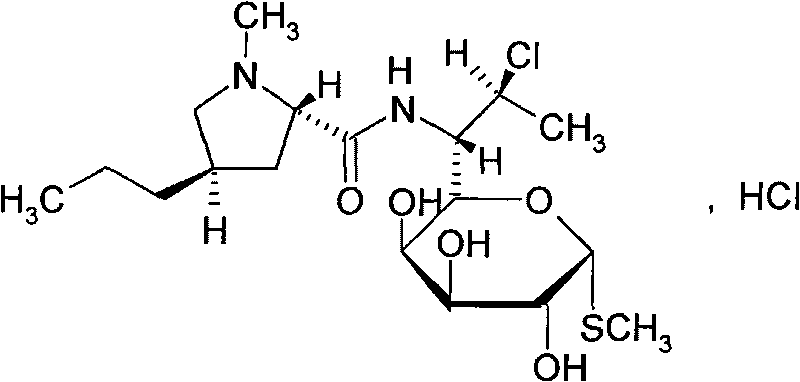
Clindamycin hydrochloride injection and preparation method thereof
A technology of clindamycin hydrochloride and clindamycin, which can be applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the risk of increasing drug safety and increase the cost of drug production , poor process reproducibility and other problems, to achieve the effect of reducing drug safety, reducing the risk of adverse reactions, and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
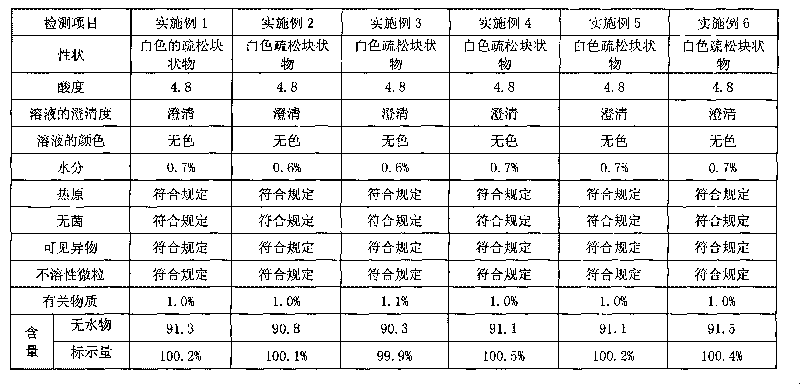
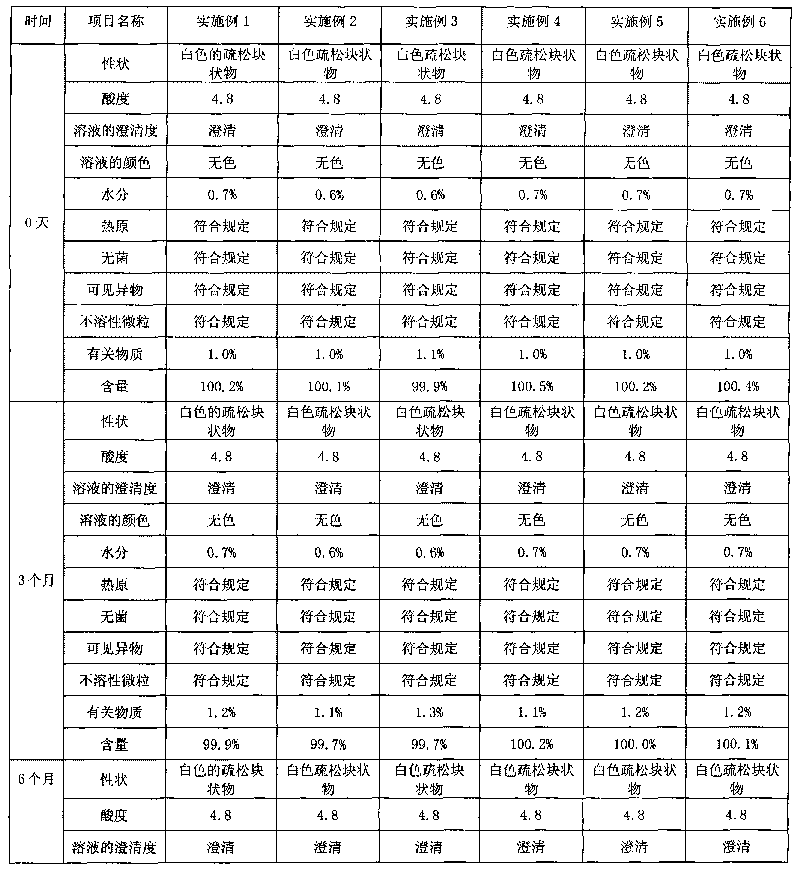
[0043] Embodiment 1: Clindamycin hydrochloride freeze-dried powder injection
[0044] Clindamycin hydrochloride 300g (calculated as clindamycin)
[0045] Acetic acid-sodium acetate buffer
[0046] Add water for injection to 1500ml
[0047]
[0048] Made 1000 pieces
[0049] Preparation process: In the clean area, first prepare the acetic acid-sodium acetate buffer solution, weigh the prescribed amount of clindamycin hydrochloride and dissolve it in an appropriate amount of water for injection, stir to dissolve, and adjust with the prepared acetic acid-sodium acetate buffer solution The pH value of the solution is 4.5, add 0.2% activated carbon to the total weight of the solution, add water for injection to the full amount, stir at room temperature for 20 minutes, and circulate for 10 minutes. The filtrate is tested for content, density, endotoxin, acidity, clarity, etc., and filled after passing the test; freeze-dried, plugged out of th...
Embodiment 2
[0050] Embodiment 2 Clindamycin hydrochloride freeze-dried powder injection
[0051] Clindamycin hydrochloride 300g (calculated as clindamycin)
[0052] Sodium acetate 7.2g
[0053] Acetic acid 4.0ml
[0054] Add water for injection to 1500ml
[0055]
[0056] Made 1000 pieces
[0057] Preparation process: In a clean area, first take an appropriate amount of water for injection and put it into a concentrated preparation tank, add the prescribed amount of clindamycin hydrochloride, and stir until completely dissolved. Dissolve the prescribed amount of acetic acid and sodium acetate in an appropriate amount of water for injection, stir to dissolve, add to the concentrated preparation tank, and stir evenly; add water for injection to the full amount, weigh 0.2% (W / V) of liquid amount of activated carbon and add to the concentrated Prepare the tank, stir and decolorize at room temperature for 25 minutes, then recirculate and stir for 15 ...
Embodiment 3
[0058] Embodiment 3 Clindamycin hydrochloride freeze-dried powder injection
[0059] Clindamycin hydrochloride 450g (calculated as clindamycin)
[0060] Acetic acid-sodium acetate buffer
[0061] Add water for injection to 2250ml
[0062]
[0063] Made 1000 pieces
[0064] Preparation process: In the clean area, first prepare the acetic acid-sodium acetate buffer solution, weigh the prescribed amount of clindamycin hydrochloride and dissolve it in an appropriate amount of water for injection, stir to dissolve, and adjust with the prepared acetic acid-sodium acetate buffer solution The pH value of the solution is 4.0, add 0.2% activated carbon, add water for injection to the full amount, stir and decolorize at room temperature for 20 minutes, recirculate and stir for 10 minutes, first coarse filter to remove carbon, and then fine filter with a 0.22 μm filter membrane; take the fine filtrate for assay , density, endotoxin, acidity, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com