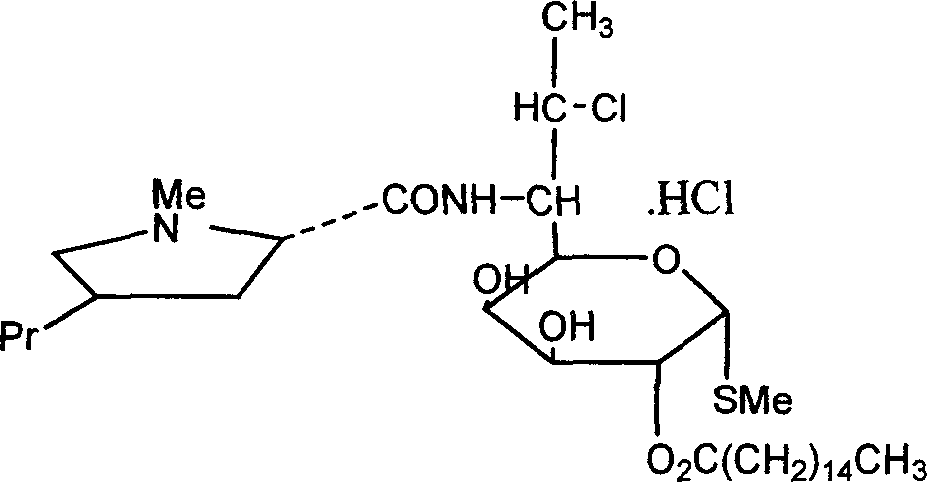
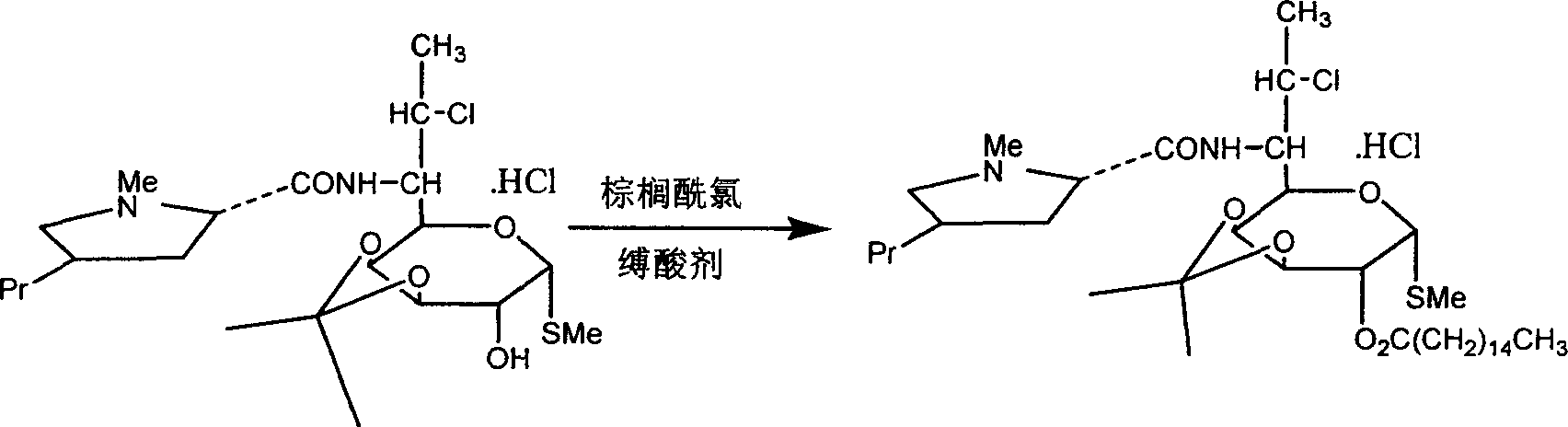
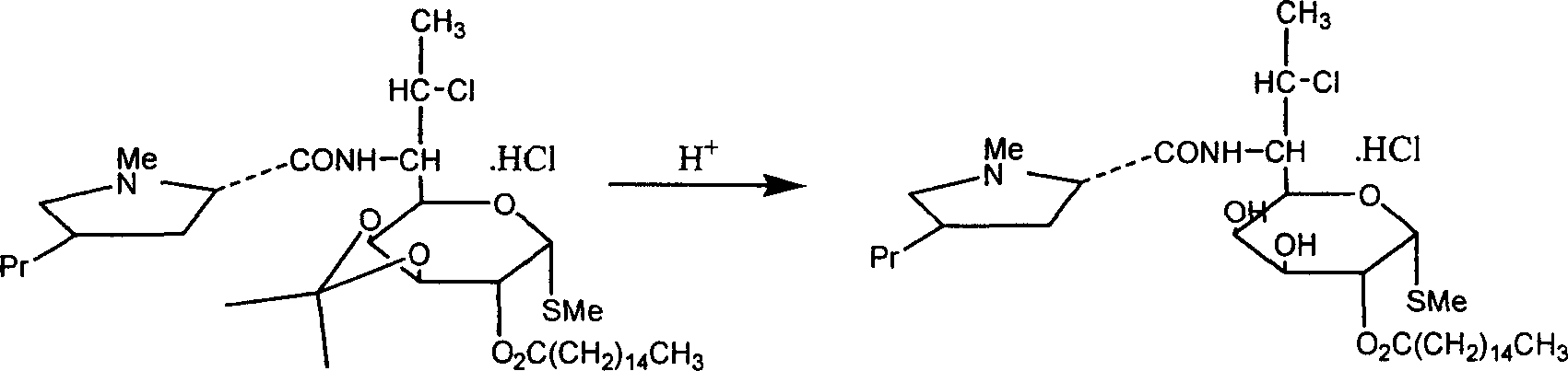
Industrial production process of palmitate of clindamycin hydrochloride
A technology of clindamycin hydrochloride and palmitate, applied in the directions of sugar derivatives, organic chemistry, anti-infective drugs, etc., can solve the problems of high toxicity, difficult to industrialized large-scale production, low purity of finished products, etc. mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 19.8kg of clindamycin hydrochloride isopropylidene and 200kg of chloroform into the reaction kettle, cool to 0°C with ice water, and add 17.5kg of palmitoyl chloride dropwise. After dropping, add 3kg triethylamine. React at 18°C for 30 minutes, then raise the temperature to 35°C and react for 3 hours to obtain about 25.0 kg of clindamycin palmitate isopropylidene hydrochloride.
[0031] Add 80kg of water to clindamycin hydrochloride palmitate isopropylidene, stir for 10min, and extract with chloroform for 3 times. Combine the organic phases, evaporate the solvent under vacuum at 0.085 MPa, add 90 kg of ethanol to the residue and reflux for 1 hour, then evaporate under vacuum at 0.085 MPa to obtain the crude clindamycin palmitate hydrochloride.
[0032] The crude product of clindamycin palmitate hydrochloride was refluxed and dissolved with 50 kg of isopropanol, the solvent was evaporated, and 78 kg of acetone was added to the residue. Stir at 40°C for 2.5 hours,...
Embodiment 2
[0034] Add 19.8kg of clindamycin hydrochloride isopropylidene and 200kg of chloroform into the reaction kettle, cool to 0°C with ice water, and add 17.5kg of palmitoyl chloride dropwise. After dropping, add 3kg of 2-picoline. React at 18°C for 30 minutes, then raise the temperature to 35°C and react for 3 hours to obtain about 25.0 kg of clindamycin palmitate isopropylidene hydrochloride.
[0035] Add 90kg of water to clindamycin hydrochloride palmitate isopropylidene, stir for 10min, and extract with n-hexane for 3 times. Combine the organic phases, evaporate the solvent to dryness under vacuum at 0.082 MPa, add 85 kg of ethanol to the residue and reflux for 1 hour, then evaporate to dryness under vacuum at 0.082 MPa to obtain the crude product of clindamycin palmitate hydrochloride.
[0036] The crude product of clindamycin palmitate hydrochloride was refluxed and dissolved with 48kg of isopropanol, the solvent was evaporated, and 78kg of acetone was added to the residue....
Embodiment 3
[0038] Add 19.8kg of clindamycin hydrochloride isopropylidene and 200kg of chloroform into the reaction kettle, cool to 0°C with ice water, and add 17.5kg of palmitoyl chloride dropwise. After dropping, add 3kg of K 2 CO 3 . React at 18°C for 30 minutes, then raise the temperature to 35°C and react for 3 hours to obtain about 25.0 kg of clindamycin palmitate isopropylidene hydrochloride.
[0039] Add 85kg of water to clindamycin hydrochloride palmitate isopropylidene, stir for 10min, and extract twice with chloroform. Combine the organic phases, evaporate the solvent to dryness under vacuum at 0.082 MPa, add 85 kg of ethanol to the residue and reflux for 1 hour, then evaporate to dryness under vacuum at 0.082 MPa to obtain the crude product of clindamycin palmitate hydrochloride.
[0040] The crude product of clindamycin palmitate hydrochloride was refluxed and dissolved with 50 kg of isopropanol, the solvent was evaporated, and 78 kg of acetone was added to the residue. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com