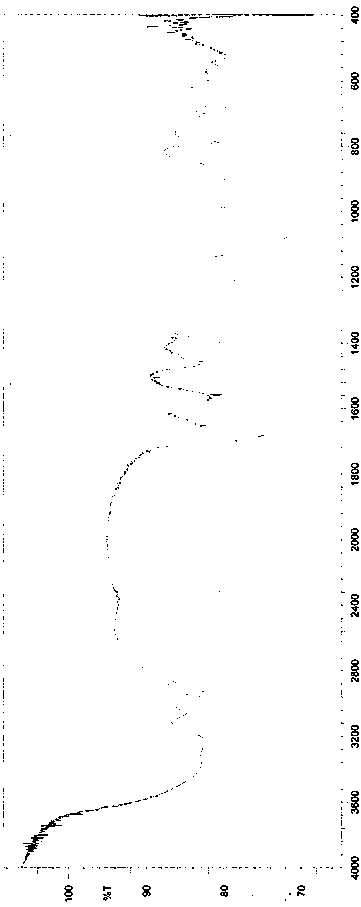
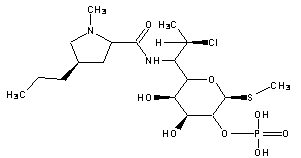
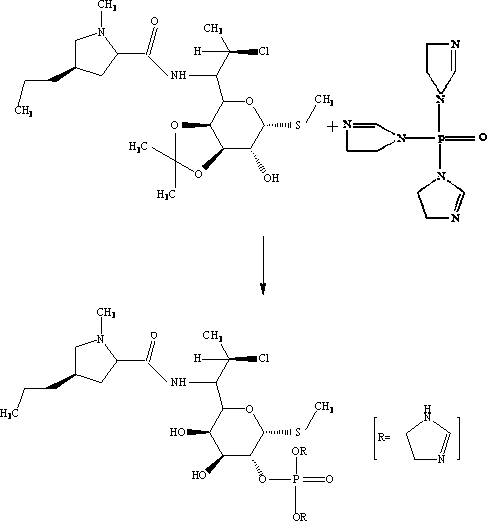
A kind of synthetic method of clindamycin phosphate
A clindamycin phosphate and synthesis method technology, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve problems such as material transfer and dripping, hidden dangers in operation safety, phosgene leakage, etc. , to achieve the effect of increasing conversion rate, reducing impurities and easy handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Chlorination reaction Put 287kg of solid phosgene into 1250kg of chloroform, stir and dissolve, add 225kg of DMF back dropwise at below 10°C, stir for 4 hours below 10°C after dropping, and then add Linco hydrochloride in batches Clindamycin 205kg, heated up to 68°C by slow heating method (5°C / h) reflux, heat preservation reaction for 8h, then cooled down to below 10°C, added alkali hydrolysis, concentrated by distillation to remove most of the chloroform, and obtained clindamycin free base Solution (contains about 200kg clindamycin free base);
[0034] (2) Ketonization reaction Add the clindamycin free base solution obtained in step (1), 1640kg of acetone, 615kg of triethyl orthoformate, and 5kg of p-toluenesulfonic acid into a reaction tank and react at 20°C for 10 hours to obtain propylidene Acetone solution of free base (contains about 210kg of propylidene free base);
[0035] (3) Phosphorylation reaction Add 225 kg of triethylamine, 123 kg of phosphorus oxychl...
Embodiment 2
[0038] (1) Chlorination reaction: put 62kg of solid phosgene into 250kg of chloroform, stir and dissolve, then add 57kg of DMF back dropwise at below 10°C, stir for 4 hours below 10°C after dropping, add Linco hydrochloride in batches Clindamycin 41kg, heated up to 65°C by slow heating method (4°C / h) reflux, kept for 10 hours, then cooled to below 10°C and hydrolyzed with alkali, distilled and concentrated to remove most of the chloroform to obtain clindamycin free base Solution (contains about 42kg clindamycin free base);
[0039] (2) Ketonization reaction Add the clindamycin free base solution obtained in step (1), 328kg of acetone, 123g of triethyl orthoformate, and 1kg of p-toluenesulfonic acid into a reaction tank and react at 50°C for 3 hours to obtain propylidene Acetone solution of free base (containing about 44kg propylidene free base);
[0040] (3) Phosphorylation reaction Add 45 kg of triethylamine, 25 kg of phosphorus oxychloride, and 41 kg of imidazole to the ace...
Embodiment 3
[0043] (1) Chlorination reaction: put 370kg of solid phosgene into 1750kg of chloroform, stir to dissolve, add 345kg of DMF back dropwise at 5°C, stir at 5°C for 4h, add lincomycin hydrochloride in batches 287kg, the temperature was raised to 71°C by slow heating method (6°C / hour) reflux, and the temperature was kept for 2 hours, then the temperature was lowered to below 10°C, hydrolyzed with alkali, most of the chloroform was removed by distillation and concentration, and clindamycin free alkali solution was obtained (contains about 290kg clindamycin free base);
[0044] (2) Ketonization reaction Add the clindamycin free base solution obtained in step (1), 1150kg acetone, 560kg triethyl orthoformate, and 5.7kg p-toluenesulfonic acid into a reaction tank and react at 35°C for 6h to obtain acetone Acetone solution of propylidene free base (containing about 300kg propylidene free base);
[0045] (3) Phosphorylation reaction Add 315 kg of triethylamine, 175 kg of phosphorus oxyc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com