Pharmaceutical composition of compound clindamycin and tazarotene lipid complex
A technology of lipid complexes and tazarotene, which is applied in the directions of drug combinations, pharmaceutical formulations, and non-active ingredients medical preparations, etc., can solve the problems of unsatisfactory effects, poor skin drug retention, and drug permeability. Not ideal and other problems, to achieve the effect of increasing the drug content, prolonging the onset time, and improving the therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1- Embodiment 3
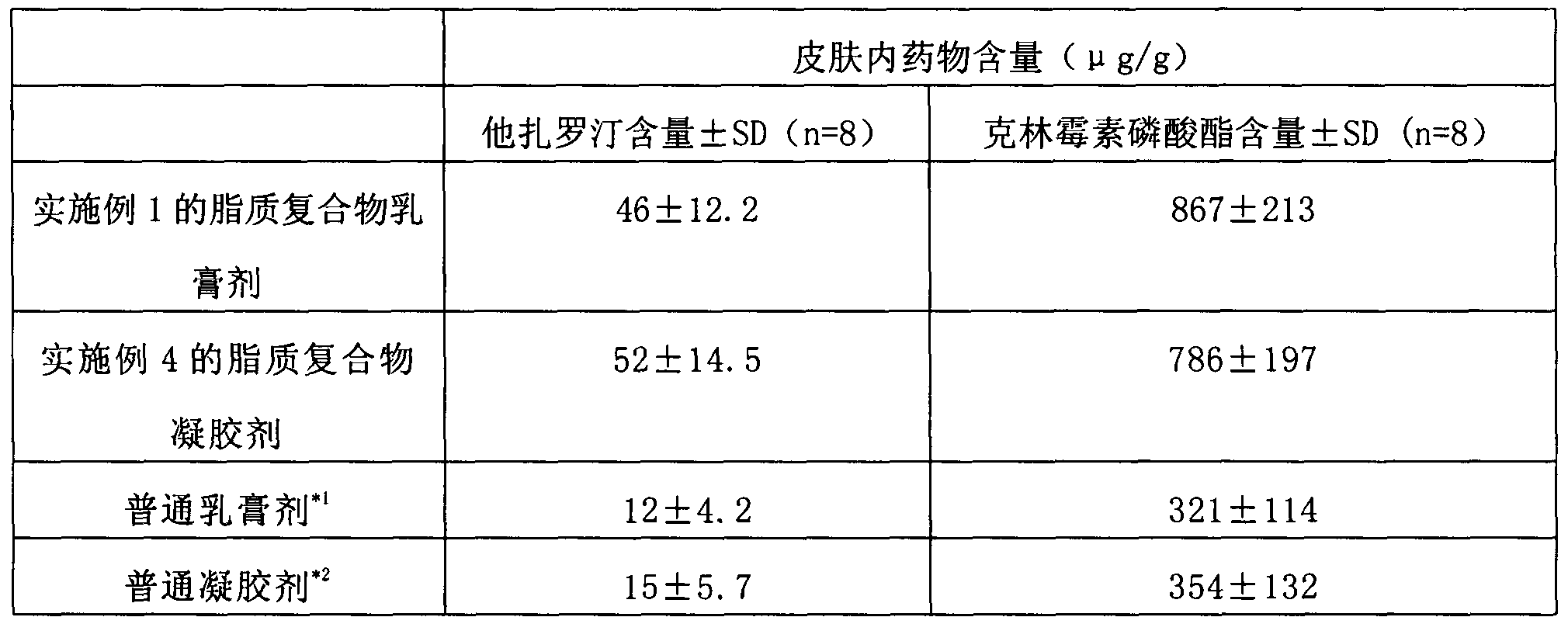
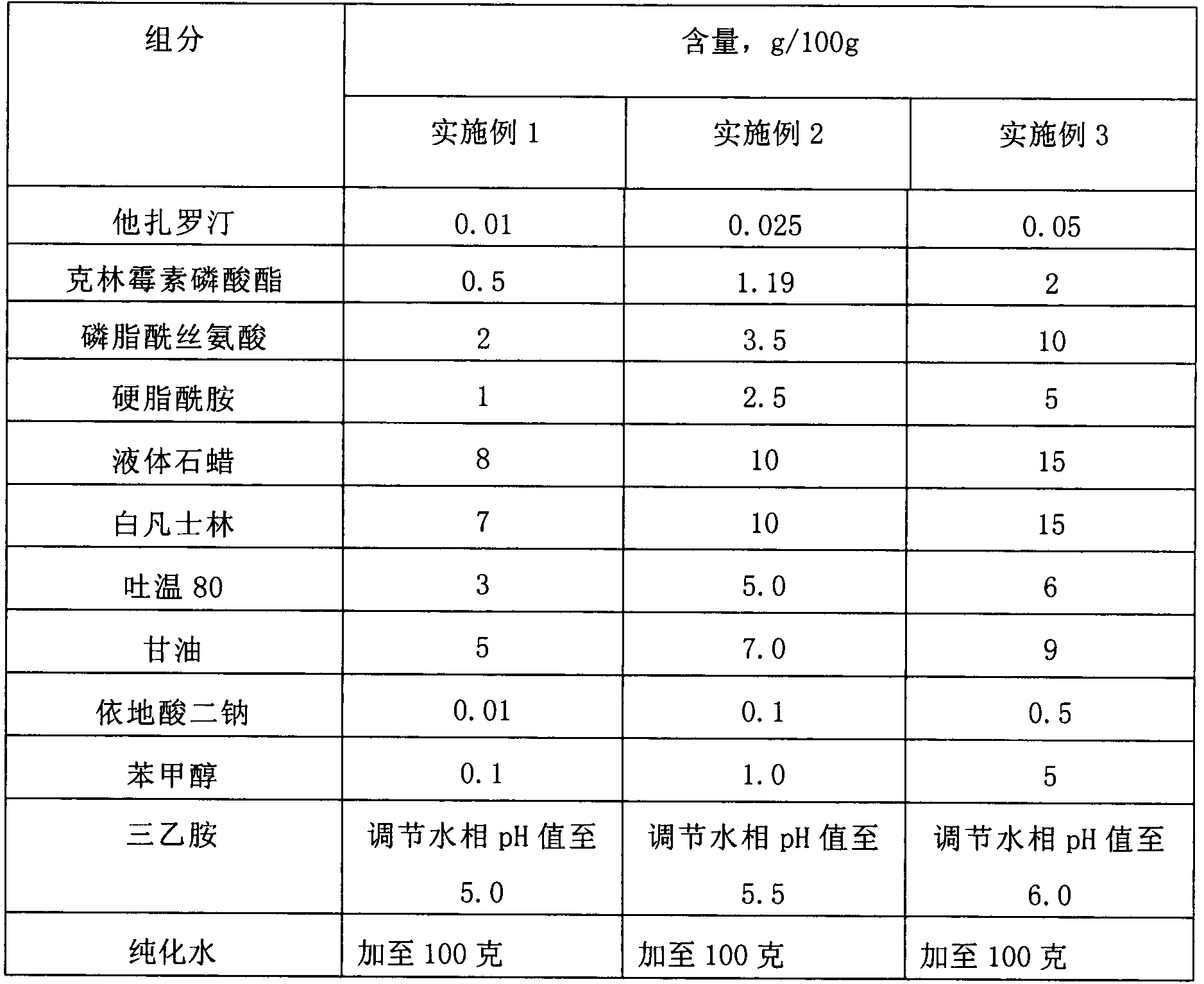
[0055] Example 1-Example 3: Compound Tazarotene Clindamycin Lipid Complex Cream
[0056]
[0057] The preparation steps are: dissolving tazarotene, phosphatidylserine and stearamide in a mixture of methylene chloride and ethanol 9-10 times the weight, the ratio of the mixture of methylene chloride and ethanol is 1:1, and then placing In a rotary evaporator, evaporate the organic solvent at 50-60°C to form a drug phospholipid film, add an appropriate volume of phosphate buffer with a pH value of 4.5-5.5, oscillate for hydration, and form a crude lipid complex suspension liquid, and then the lipoplex suspension was homogenized twice through high-pressure milk, and finally passed through a 100nm polycarbonate membrane to obtain a drug-loaded lipoplex suspension.
[0058] Take liquid paraffin and white petrolatum as the oil phase matrix and heat to 80°C to melt; take the above-mentioned drug-loaded lipid complex suspension, dilute with purified water, add Tween 80, glycerin, an...
Embodiment 4- Embodiment 6
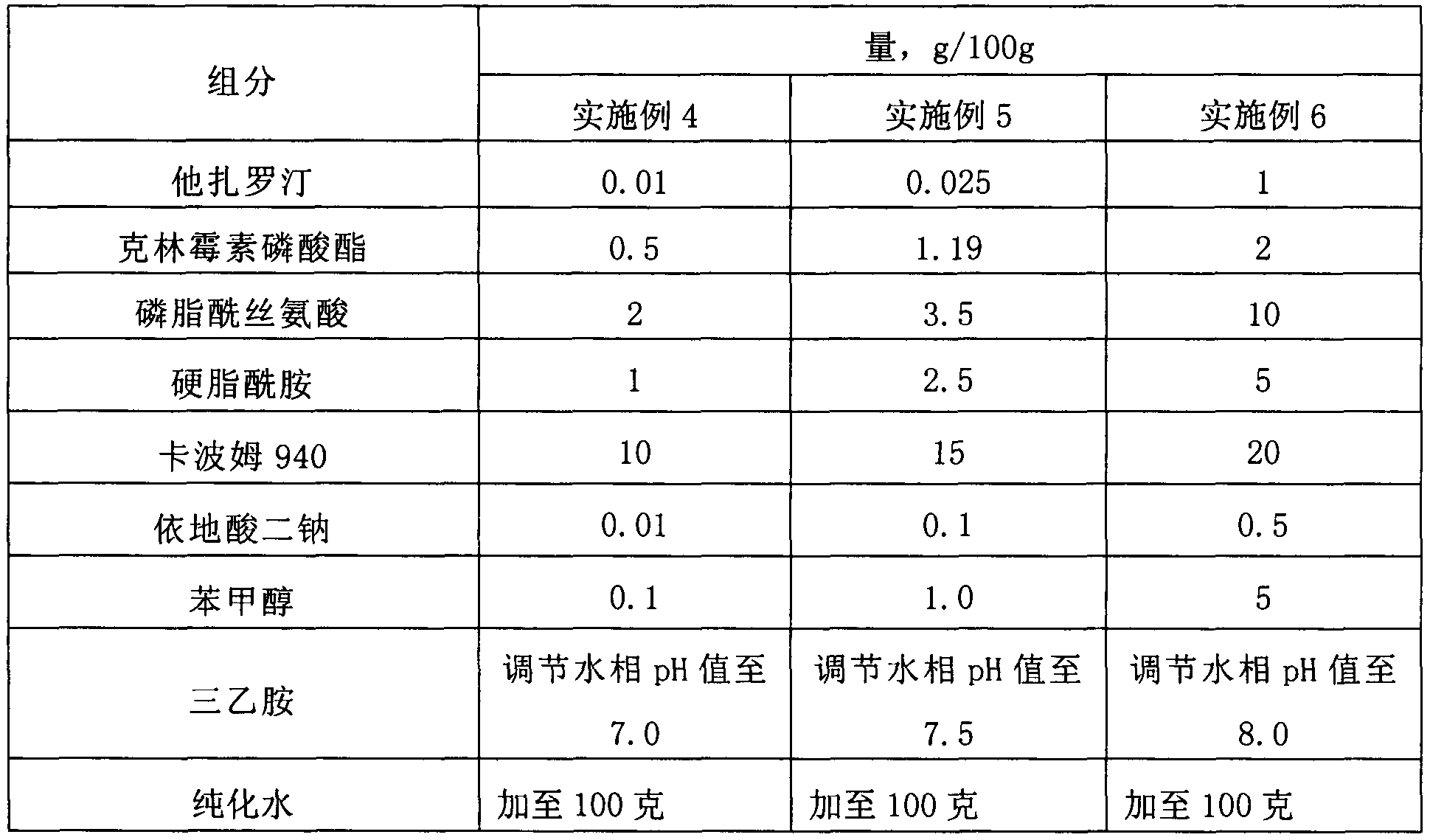
[0059] Example 4-Example 6: Compound Tazarotene Clindamycin Lipid Complex Gel
[0060]
[0061] The preparation steps are: dissolving tazarotene, phosphatidylserine and stearamide in a mixture of methylene chloride and ethanol 9-10 times the weight, the ratio of the mixture of methylene chloride and ethanol is 1:1, and then, Place in a rotary evaporator, evaporate the organic solvent at 50-60°C to form a drug phospholipid film, add an appropriate volume of phosphate buffer with a pH value of 4.5-5.5, oscillate to hydrate, and form a lipoplex suspension solution, and then the lipoplex suspension was passed through high-pressure milk twice, and finally passed through a 100nm polycarbonate membrane.
[0062] Take the drug-encapsulated lipid complex suspension prepared above, dilute it with purified water, add Carbomer 940 gel matrix to disperse and fully swell, adjust the pH value to 7-8 with triethylamine, and add in turn Disodium diacetate and benzyl alcohol are stirred eve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com