Impurity analysis preparation method for clindamycin
A technology for clindamycin and impurity analysis, applied in the fields of analytical chemistry and pharmaceutical analytical chemistry, can solve the problems of reducing the limit of impurities, seldom considering the adverse effects of impurities on drug safety, and the control of impurities is not comprehensive and accurate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] LC-MS Determination of Clindamycin API and Crude Products
[0089] Liquid-mass spectrometer: HPLC Waters 2486, MS Waters micromass ZQ 4000. Chromatographic column: Diamonsil C18 (5 μ 250 × 4.6mm); mobile phase is acetonitrile-tetrahydrofuran-water-formic acid (18%: 3%: 79%: 0.2%), ammonia water adjusts the pH value to 5.45; column temperature is room temperature; detection wavelength 210nm; flow rate 1.0mL / min, split into mass spectrometer. The mass spectrometry conditions are electrospray ionization source positive ion (ESI+) detection mode; source temperature 80°C; cone voltage 35v.
[0090] LC-MS detection of raw materials
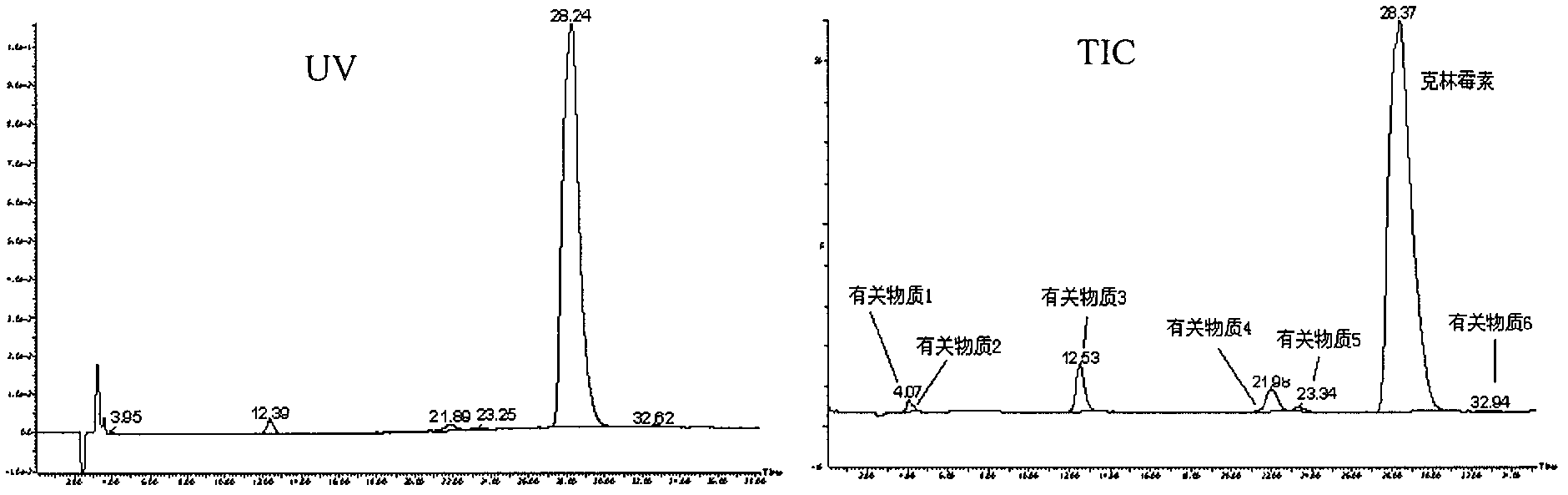
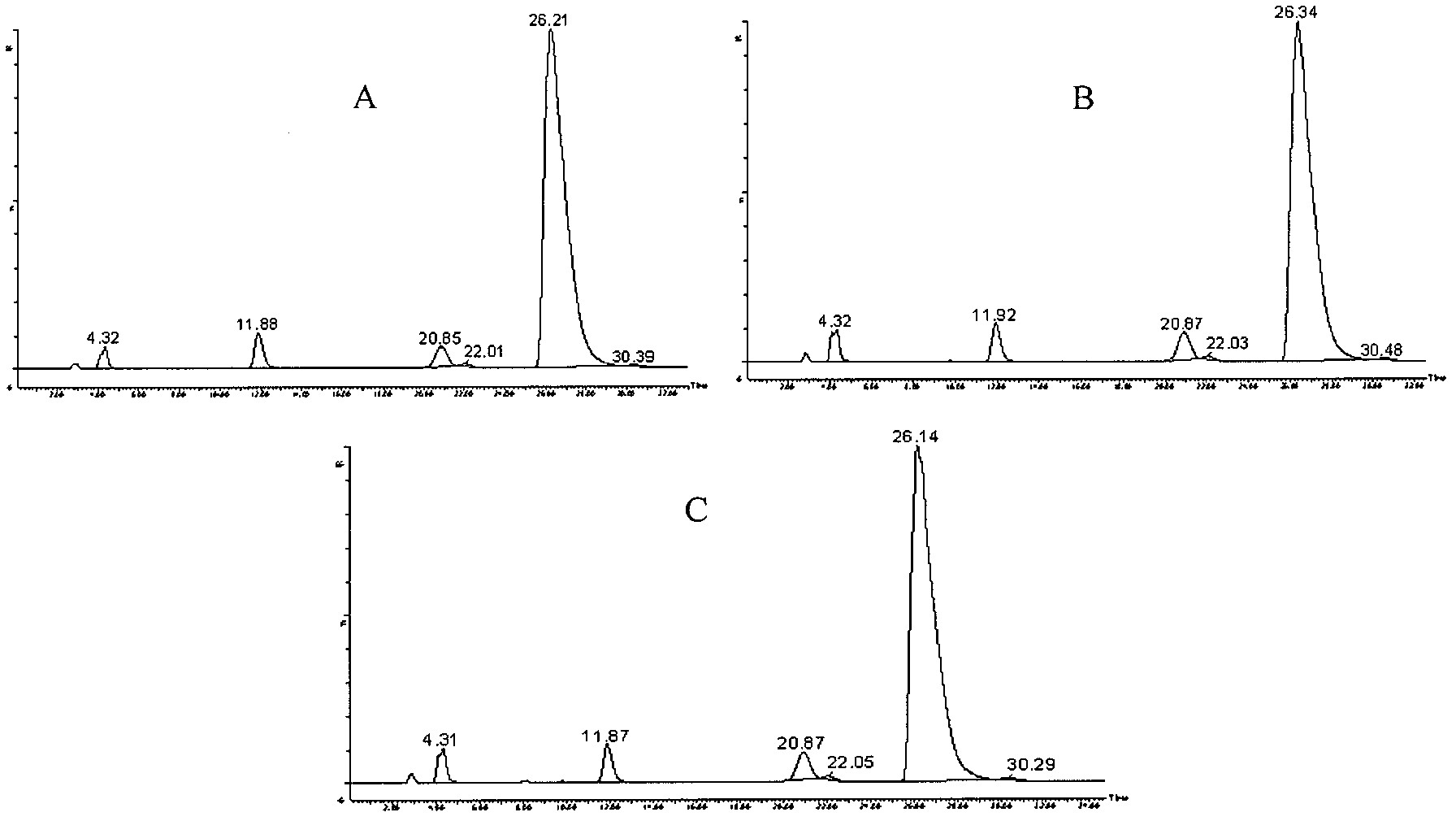
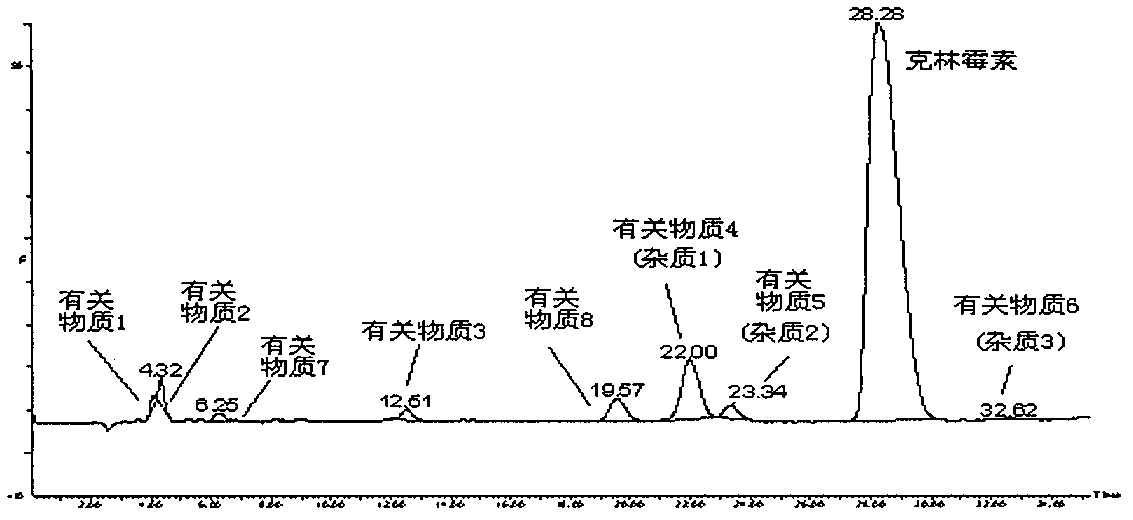
[0091] Take the mobile phase of the raw material medicine with batch number 090303×7 and dissolve it into a solution with a concentration of 2 mg / mL, and the injection volume is 20 μL. LC-MS detection results such as figure 1 shown.
[0092] Six related substances except clindamycin in clindamycin hydrochloride API were detected by liqui...
Embodiment 2
[0139] Structural identification of target impurities
[0140] The obtained pure products of the three target impurities were determined by high-resolution mass spectrometry, and then the structures of the three target impurities were determined by H-NMR, C-NMR and two-dimensional DEPT, HMBC, HMQC, COZY and NOESY spectra. The instruments used are Micromass Q-TOF mass spectrometer and American Varian nuclear magnetic resonance spectrometer (400MHz). The following structural formula is the structure of clindamycin, and Table 4 is the data of clindamycin nuclear magnetic resonance spectrum.
[0141]
[0142] Clindamycin
[0143] Table 4. Clindamycin 1 H-NMR spectrum, 13 C-NMR spectrum, HMQC spectrum, COZY spectrum, NOESY spectrum assignment
[0144]
[0145] There are four chiral centers in the structure of clindamycin, and their configurations are 6S, 7S, 1’S, 3’R.
[0146] Structural identification of impurity 1
[0147] Instrument: Micromass Q-TOF mass spectro...
Embodiment 3
[0190] Bacteriostasis experiment of clindamycin hydrochloride and impurities 1, 2 and 3 of the present invention
[0191] The tests used to determine the effectiveness of antibacterial drugs in inhibiting bacterial growth in vitro are called bacteriostatic tests. In this experiment, the antibacterial activity of three related substances whose apparent content exceeds 0.1% in clindamycin hydrochloride bulk drug was investigated. active control.
[0192] Preparation of the test solution
[0193] Clindamycin hydrochloride (090303×7 batches, Zhejiang Tiantai Pharmaceutical Co., Ltd.): 1.091mg, dissolved in 1mL water;
[0194] Impurity 1 (i.e. 7-epiclindamycin): 1.049mg, dissolved in 0.5mL water;
[0195] Impurity 2: 1.200mg, dissolved in 0.5mL water;
[0196] Impurity 3: 1.139 mg, dissolved in 0.4 mL of water.
[0197] Experimental strain
[0198] Staphylococcus aureus (Gram-positive bacteria), Bacillus subtilis (bacteria), Candida albicans (fungi); provided by the Depar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com