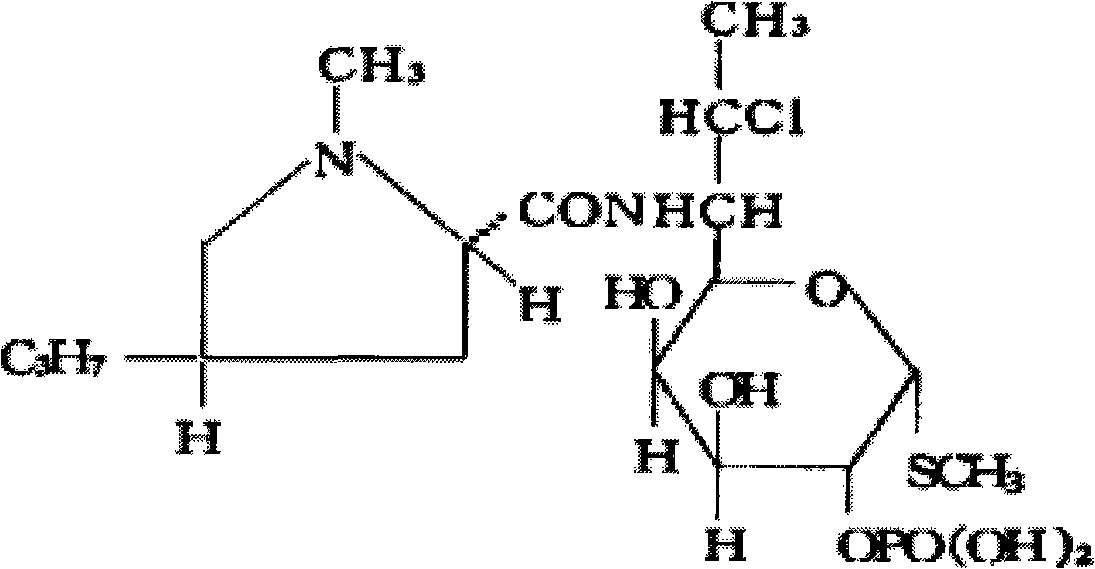
Preparation method of clindamycin phosphate powder for injection
A technology for clindamycin phosphate and injection, which is applied in the field of medicine, can solve the problems of accelerating the decomposition of clindamycin phosphate, increasing production equipment requirements, and high production costs, so as to avoid decomposition and damage, increase unit output, and improve Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A preparation method of clindamycin phosphate for injection, comprising the following steps:
[0041] 1. Preparation of Clindamycin Phosphate Sterile Solution:
[0042] Take 500g of clindamycin phosphate, add 1000mL of 1M sodium hydroxide solution and stir to dissolve (the pH value of the solution is about 6.8 at this time), add activated carbon for decolorization for 30min, coarse filter to remove carbon, and then use a 0.22μm microporous membrane Filter until clear.
[0043] 2. Preparation of Clindamycin Phosphate Sterile Powder:
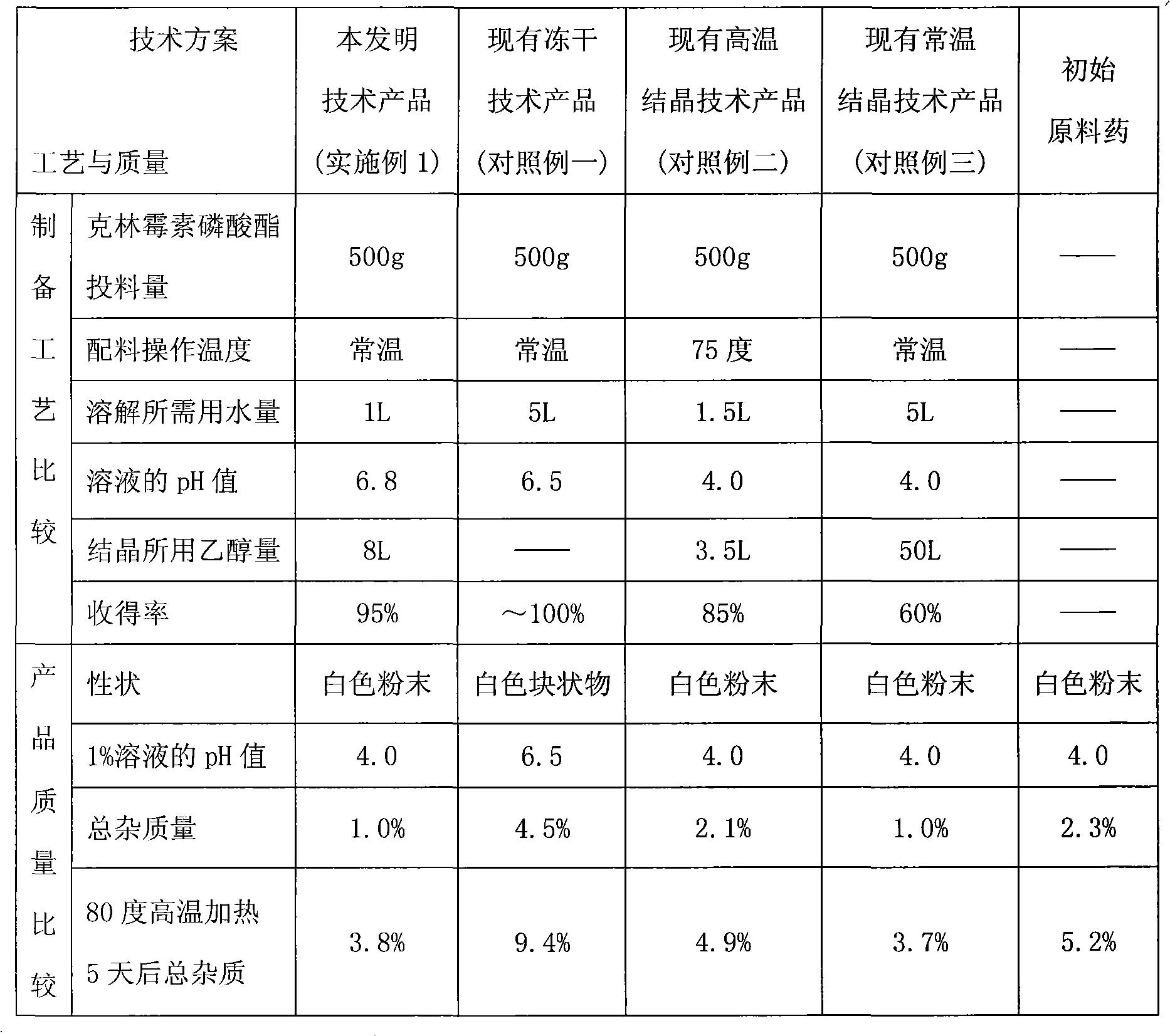
[0044] In the sterile environment of the production control area, add the sterile solution of clindamycin phosphate in step 1 to 1000ml of 1M hydrochloric acid ethanol solution after sterile filtration and adjust the pH to 4, then add 8000mL of sterile filtered ethanol And continue to stir for crystallization (equivalent to clindamycin phosphate: sodium hydroxide: water: ethanol ratio of 1: 0.08: 2: 16), filter, wash, and drain. Dry unde...
Embodiment 2
[0048] A preparation method of clindamycin phosphate for injection, comprising the following steps:
[0049] 1. Preparation of Clindamycin Phosphate Sterile Solution:
[0050] Take 500g of clindamycin phosphate, add 1500mL of 0.5M sodium hydroxide solution and stir to dissolve (the pH value of the solution is about 6.0 at this time), add activated carbon for decolorization for 30min, coarsely filter to remove carbon, and then use a 0.22μm microporous filter Membrane filtration until clear.
[0051] 2. Preparation of Clindamycin Phosphate Sterile Powder:
[0052] Under the sterile environment of the production control area, add the above-mentioned sterile solution of clindamycin phosphate to 750ml of 1M nitric acid ethanol solution after aseptic filtration to adjust the pH to 4, then add 15000mL of ethanol through aseptic filtration and Stir continuously to crystallize (equivalent to clindamycin phosphate: sodium hydroxide: water: ethanol at 1:0.06:3:30), filter, wash, and dr...
Embodiment 3
[0056] A preparation method of clindamycin phosphate for injection, comprising the following steps:
[0057] 1. Preparation of Clindamycin Phosphate Sterile Solution:
[0058] Take 500g of clindamycin phosphate, add 750mL of 2M sodium hydroxide solution and stir to dissolve (the pH value of the solution is about 8 at this time), add activated carbon for decolorization for 30min, coarse filter to remove carbon, and then use a 0.22μm microporous membrane Filter until clear.
[0059] 2. Preparation of Clindamycin Phosphate Sterile Powder:
[0060] Under the sterile environment of the production control area, add the above-mentioned aseptic clindamycin phosphate solution to 750ml of 1M sulfuric acid ethanol solution after aseptic filtration to adjust the pH to 4, then add 1500mL of ethanol through aseptic filtration and Stir continuously to crystallize (equivalent to clindamycin phosphate: sodium hydroxide: water: ethanol ratio of 1: 0.12: 1.5: 3), filter, wash, and drain. Dry ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com