Construction of paclitaxel-oleic acid small-molecular prodrug self-assembled nanoparticles
A technology of self-assembled nanoparticles and paclitaxel, which is applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem that PTX-DHA does not show advantages in curative effect, paclitaxel breaks, drug is easy to Leakage and other problems, to reduce adverse reactions, the preparation method is simple and easy, easy to modify the effect of the surface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the synthesis of paclitaxel-oleic acid prodrug (PTX-OA) linked by ester bond
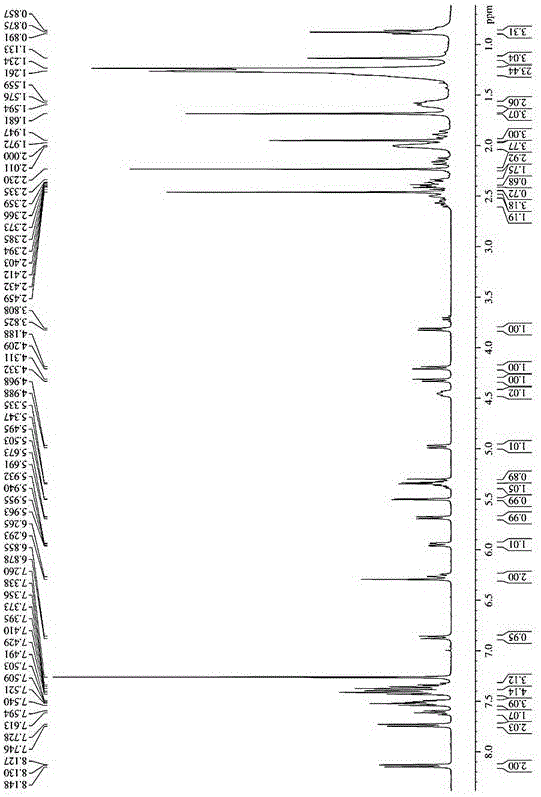
[0039] Oleic acid (OA) was dissolved in a small amount of dichloromethane, under the catalysis of DCC and DMAP, in N 2 Under protection, ice bath 2-4h, and then with paclitaxel at 25 ℃ N 2 The reaction was carried out for 24-48 hours under protection, and the white powder prodrug was obtained by separation and purification. Determination by NMR 1 H-NMR hydrogen spectrum is determined the structure of prodrug in embodiment 1, and the solvent of selection is CDCl 3 , the result is as figure 1 , the spectral analysis results are as follows:
[0040] 1 H-NMR (400MHz, CDCl 3 )δ:8.13(t,2H),7.74(d,2H),7.61(m,1H),7.59(m,3H),7.42(d,4H),7.40(m,3H),6.87(d,1H , J=9.2Hz, -NH-), 6.29(t, 2H, 10-H, 13-H), 5.95(dd, 1H, J=3.2Hz, J=6.0Hz, 3'-H), 5.69( d,1H,J=7.2Hz,2-H),5.50(d,1H,J=3.2Hz,2'-H),5.34(m,2H,-CH=CH-),4.98(d,1H, J=8.0Hz, 5-H), 4.45(t, 1H, 7-H), 4.31(d, 1H, J=8.4Hz, 20α-H), 4.21(...
Embodiment 2
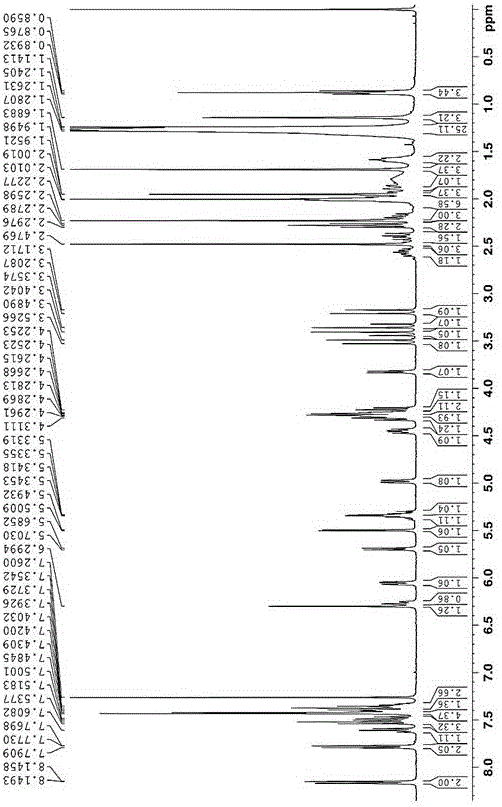
[0041] Example 2: Synthesis of Paclitaxel-oleic acid prodrug (PTX-S-OA) linked by redox double-sensitive monosulfide bond
[0042] Put ethylene glycol in a 50mL three-necked bottle, add an appropriate amount of p-toluenesulfonic acid to the eggplant-shaped bottle, N 2 Protected, heated to 100-120 ° C, slowly dripping the oleic acid solution dissolved in an appropriate amount of toluene into the reaction bottle, and reacted for 2-4 hours. After the reaction is over, let the layers stand, extract with toluene until the ethylene glycol layer TLC has no product point, combine the toluene layers, and then use saturated NaHCO 3 The solution was washed until neutral, dried over anhydrous sodium sulfate, filtered, evaporated to dryness, separated and purified to obtain (Z)-oleic acid-2-hydroxyethyl ester. Put an appropriate amount of 2,2'-thiodiacetic anhydride and a small amount of triethylamine in a 25mL eggplant-shaped bottle, add 10-15mL CH 2 Cl 2 , joining HOBt and EDCI, N 2 ...
Embodiment 3
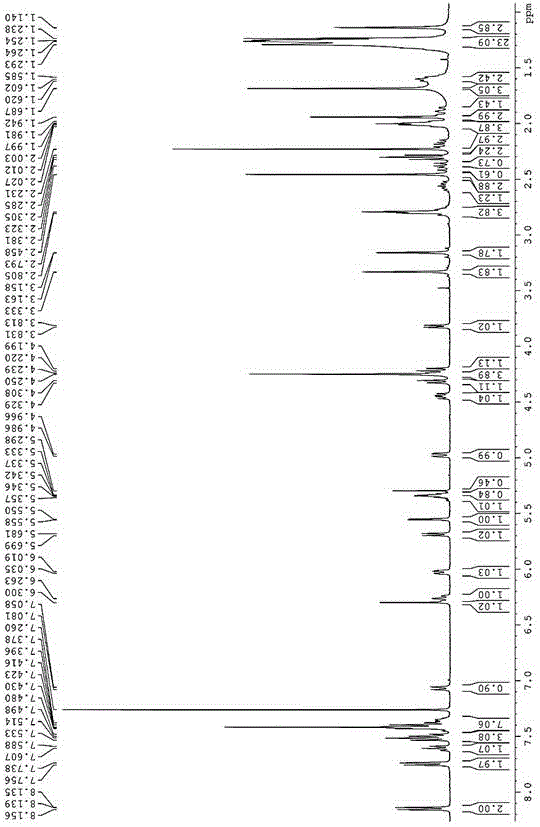
[0045] Example 3: Synthesis of Paclitaxel-Oleic Acid Prodrug (PTX-2S-OA) Linked by Redox Double Sensitive Spacer Disulfide Bond
[0046] Put ethylene glycol in a 50mL three-necked bottle, add an appropriate amount of p-toluenesulfonic acid to the eggplant-shaped bottle, N 2 Protected, heated to 100-120 ° C, slowly dripping the oleic acid solution dissolved in an appropriate amount of toluene into the reaction bottle, and reacted for 2-4 hours. After the reaction is over, let the layers stand, extract with toluene until the ethylene glycol layer TLC has no product point, combine the toluene layers, and then use saturated NaHCO 3 The solution was washed until neutral, dried over anhydrous sodium sulfate, filtered, evaporated to dryness, separated and purified to obtain (Z)-oleic acid-2-hydroxyethyl ester. Put an appropriate amount of 1,2-ethylenedithiodiacetic anhydride and a small amount of triethylamine in a 25mL eggplant-shaped bottle, add 10-15mL CH 2 Cl 2 , joining HOBt ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com