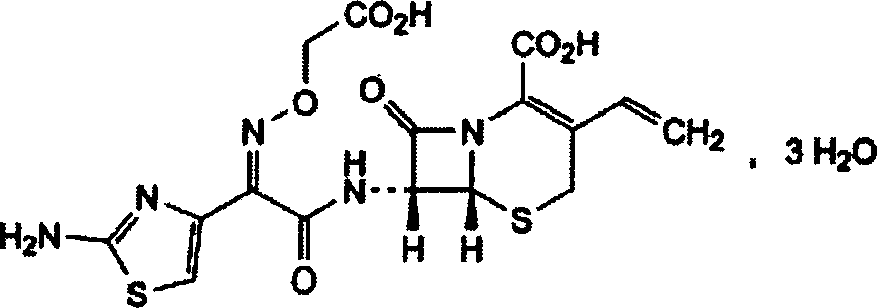
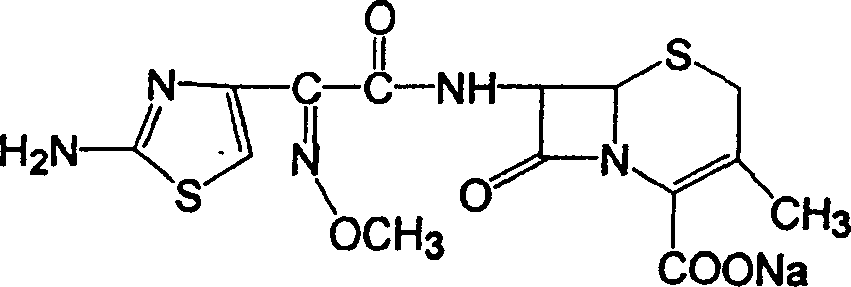
Compound medicinal preparation for treating pneumonia infection disease and its preparation method
A technology for pharmaceutical preparations and pulmonary infections, which is applied in the field of anti-infective compound pharmaceutical preparations and its preparation, can solve the problems of large dosage, inaccurate dosage, and influence on the treatment effect, and achieve small dosage, convenient clinical application, and effective The effect of increasing strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of cefixime ambroxol sheet
[0029] prescription:
[0030] Cefixime 100g
[0031] Ambroxol Hydrochloride 60g
[0032] Starch 25g
[0033] Microcrystalline Cellulose 50g
[0034] Diluted Hydroxypropyl Cellulose 12.5g
[0036] Magnesium Stearate 2.5g
[0037] Made into 1000 pieces
[0038] Preparation process: Weigh cefixime, ambroxol hydrochloride, starch, microcrystalline cellulose, and low-substituted hydroxypropyl cellulose according to the above prescription, pass through a 80-mesh sieve after crushing, mix evenly, and add 10% starch slurry to prepare Soft material, pass through a 20-mesh sieve to make wet granules, dry at 50°C for 3 hours, add magnesium stearate, pass through a 18-mesh sieve for granulation, and compress into tablets, each weighing about 0.25g.
Embodiment 2
[0039] Embodiment 2: the preparation of cefixime ambroxol dispersible tablet
[0040] prescription:
[0041] Cefixime 100g
[0042] Ambroxol Hydrochloride 60g
[0043] Lactose 37.5g
[0044] Microcrystalline Cellulose 36g
[0045] Cross-linked polyvinylpyrrolidone 15g
[0046] 10% polyvinylpyrrolidone solution 30ml
[0047] Sodium Lauryl Sulfate 1.5g
[0048] Made into 1000 pieces
[0049] Preparation process: Weigh cefixime, ambroxol hydrochloride, lactose, microcrystalline cellulose, and cross-linked polyvinylpyrrolidone according to the above prescription, pass through an 80-mesh sieve after crushing, mix well, and add 10% polyvinylpyrrolidone solution to prepare Soft material, pass through a 30-mesh sieve to make wet granules, dry at 50°C for 3 hours, add sodium lauryl sulfate, pass through a 20-mesh sieve for granulation, and press into tablets, the weight of the tablet is about 0.25g.
Embodiment 3
[0050] Embodiment 3: the preparation of cefixime ambroxol capsule
[0051] prescription:
[0052] Cefixime 100g
[0053] Ambroxol Hydrochloride 60g
[0054] Starch 25g
[0055] Microcrystalline Cellulose 50g
[0056] Diluted Hydroxypropyl Cellulose 12.5g
[0057] 10% starch slurry 25ml
[0058] Magnesium Stearate 2.5g
[0059] Make 1000 capsules
[0060] Preparation process: Weigh cefixime, ambroxol hydrochloride, starch, microcrystalline cellulose, and low-substituted hydroxypropyl cellulose according to the above prescription, pass through a 80-mesh sieve after crushing, mix evenly, and add 10% starch slurry to prepare Soft material, pass through a 20-mesh sieve to make wet granules, dry at 50°C for 3 hours, add magnesium stearate, pass through a 18-mesh sieve for granulation, fill the capsules, the weight of the capsules is about 0.25g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com