Crystallization method of cefixime
A cefixime crystallization technology, which is applied in the preparation of cephalosporins and the crystallization field of cefixime, can solve the problem of unsuitable cefixime capsule subpackage, etc., and achieve the effect of high bulk density and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
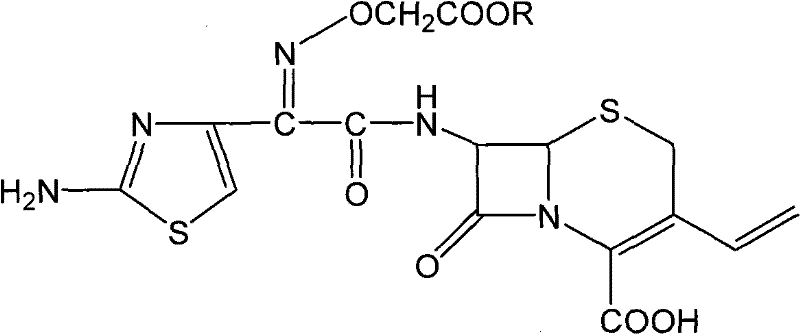
[0036] 10 grams of cefixime intermediate A (R is -C (CH 3 ) 3 ) in 100ml of water, cooled to 0-10°C, adding 60ml of 20wt% sodium hydroxide solution and stirring for 10 minutes, adding 36wt% hydrochloric acid to adjust the pH of the solution to 6-7 to obtain cefixime sodium salt solution; Add 1 g of activated carbon to the solution, stir and decolorize for 20 minutes, filter out the activated carbon and wash it with 20ml of water, combine the filtrate and the carbon washing solution to obtain a clear cefixime sodium salt solution; keep the temperature of the solution at 15-20°C, add dropwise 18wt% hydrochloric acid until the pH of the solution is 3.0 to 3.3, stirring to precipitate cefixime crystals, while stirring, monitor the pH of the system with an online pH meter, and maintain the pH of the system by adding 18wt% hydrochloric acid dropwise and controlling the rate of addition of 18wt% hydrochloric acid In the range of 3.0 to 3.3, continue until the pH of the solution is i...
Embodiment 2
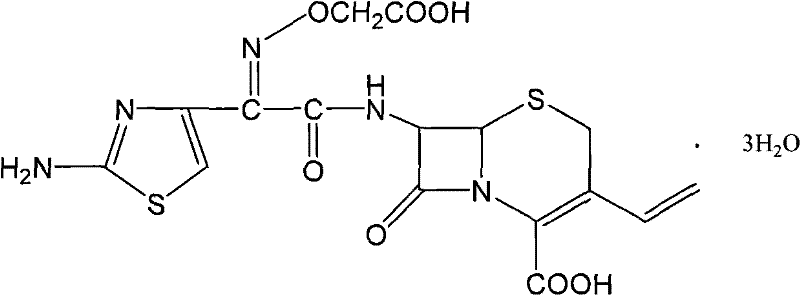
[0039] Disperse 10 grams of cefixime (content 94%, yellow in color) in 180ml of water and 50ml of ethanol, cool to 0-10°C, add sodium bicarbonate until the cefixime dissolves (solution pH=6.5-7.5), and then Add 0.5 g of active carbon, stir and decolorize for 20 minutes, filter out active carbon and wash with 20 ml of water, combine the filtrate and the carbon washing liquid to obtain a clear cefixime salt solution; add 30 ml of isopropanol to this solution, and keep the solution temperature at 15 ~25°C, add 10wt% hydrochloric acid dropwise until the pH of the solution is 3.0~3.3, stir to precipitate cefixime crystals, and monitor the pH of the system with an online pH meter while stirring, and use dropwise addition of 10wt% hydrochloric acid and control the dropping speed of 10wt% hydrochloric acid Keep the pH of the solution in the range of 3.0 to 3.3 by means of the method, and continue until the pH of the solution does not fluctuate upwards in the range of 3.0 to 3.3 for 0.5...
Embodiment 3
[0042] 10 grams of cefixime intermediate A (R is -CH 3 ) in 100ml of water, cooled to 0-10°C, adding 60ml of 20wt% sodium hydroxide solution and stirring for 10 minutes, adding 36wt% hydrochloric acid to adjust the pH of the solution to 5.5-6.5 to obtain cefixime sodium salt solution; Add 2 grams of activated carbon to the solution, stir and decolorize for 20 minutes, filter the activated carbon and wash it with 20 ml of water, combine the filtrate and the carbon washing solution to obtain a clear cefixime sodium salt solution; add 4 ml ethyl acetate to the solution, and keep the solution temperature At 25-30°C, add 18wt% hydrochloric acid dropwise until the pH of the solution is 3.3-3.5, stir to precipitate cefixime crystals, and monitor the pH of the system with an online pH meter while stirring, and use dropwise addition of 18wt% hydrochloric acid and control 18wt% Keep the pH of the solution in the range of 3.3 to 3.5 by means of hydrochloric acid drop rate, and continue u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com