Cefixime crystal, preparation method thereof and tablet composition containing same
A technology of cefixime and a composition, applied in the field of pharmaceutical preparations, can solve the problems of high sales price, increased adverse reactions of oxidation or degradation products, and increased economic burden of drugs for low-income groups, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation of embodiment 1 cefixime crystal
[0047] (1) Take 10Kg cefixime by weighing, be dissolved in tetrahydrofuran, obtain the cefixime tetrahydrofuran solution that concentration is 0.10g / ml;
[0048] (2) Add pure water dropwise to the tetrahydrofuran solution of cefixime at a stirring speed of 180 r / min until the solution is no longer clear, and maintain the temperature of the solution at 30°C;
[0049] (3) Add an organic mixed solution of ethanol and ether to the solution obtained in step 2 at a stirring speed of 75r / min; the flow rate is 12ml / min; the volume ratio of ethanol and ether in the organic mixed solution is: 1: 3; The volume ratio of the organic mixed solution to THF is 1:1.
[0050] (4) Stand still, grow crystals at 12° C. for 4 hours, filter, wash the filter cake with 65% ethanol solution, and dry in vacuum to obtain cefixime crystals.
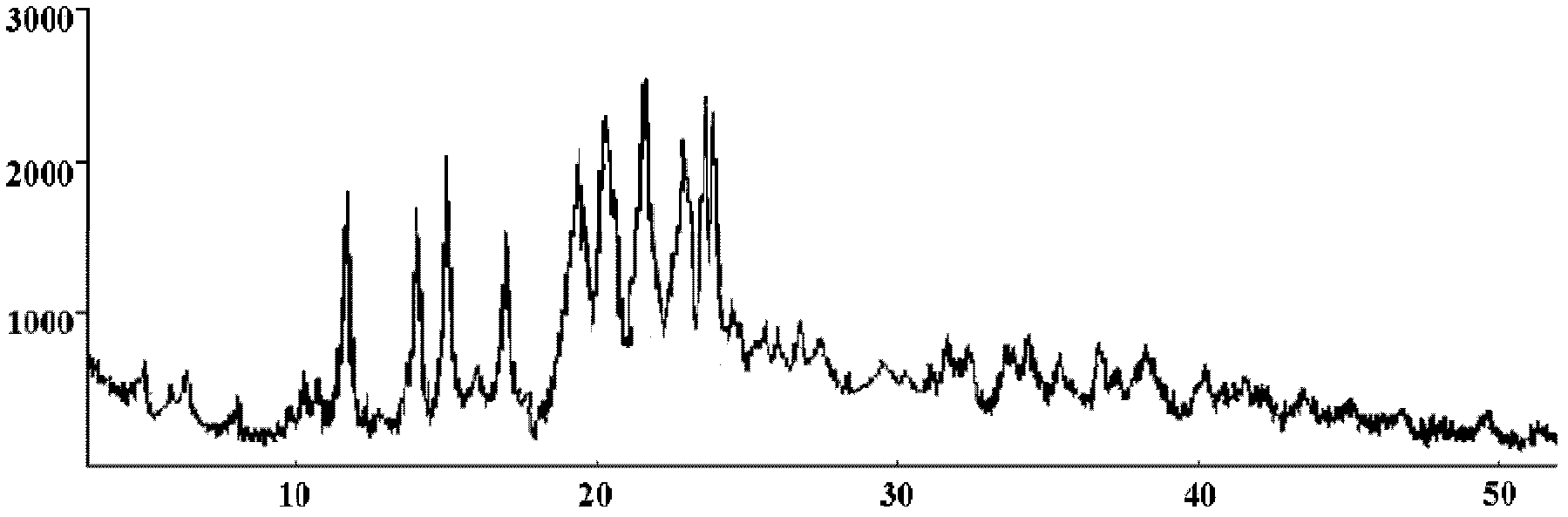
[0051] Such as figure 1 As shown, the prepared cefixime crystals use Cu-K α rays to measure the characte...
Embodiment 2
[0052] The preparation of embodiment 2 cefixime crystals
[0053] (1) Take 10Kg cefixime by weighing, be dissolved in tetrahydrofuran, obtain the cefixime tetrahydrofuran solution that concentration is 0.06g / ml;
[0054] (2) Add pure water dropwise to the tetrahydrofuran solution of cefixime at a stirring speed of 160 / min until the solution is no longer clear, and maintain the temperature of the solution at 28°C;
[0055] (3) Add an organic mixed solution of ethanol and ether to the solution obtained in step 2 at a stirring speed of 60r / min; the flow rate is 10ml / min; the volume ratio of ethanol and ether in the organic mixed solution is: 5:18; The volume ratio of the organic mixed solution to THF is 2:3.
[0056] (4) Stand still, grow crystals at 10° C. for 3 hours, filter, wash the filter cake with 60% ethanol solution, and dry in vacuum to obtain cefixime crystals.
[0057] Such as figure 1 As shown, the prepared cefixime crystals use Cu-K α rays to measure the character...
Embodiment 3
[0058] The preparation of embodiment 3 cefixime crystals
[0059] (1) Take 10Kg cefixime by weighing, be dissolved in tetrahydrofuran, obtain the cefixime tetrahydrofuran solution that concentration is 0.16g / ml;
[0060] (2) Add pure water dropwise to the tetrahydrofuran solution of cefixime at a stirring speed of 200 r / min until the solution is no longer clear, and maintain the temperature of the solution at 32°C;
[0061] (3) Add an organic mixed solution of ethanol and ether to the solution obtained in step 2 at a stirring speed of 90r / min; the flow rate is 15ml / min; the volume ratio of ethanol and ether in the organic mixed solution is: 5: 11; The volume ratio of the organic mixed solution to THF is 4:3.
[0062] (4) Stand still, grow crystals at 15° C. for 5 hours, filter, wash the filter cake with 70% ethanol solution, and dry in vacuum to obtain cefixime crystals.
[0063] Such as figure 1 As shown, the prepared cefixime crystals use Cu-K α rays to measure the charac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com