Adeno-associated virus vector and application thereof
A carrier and virus particle technology, which is applied in the fields of genetic engineering and gene therapy, can solve the problems that safe and effective carriers have not yet been identified
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
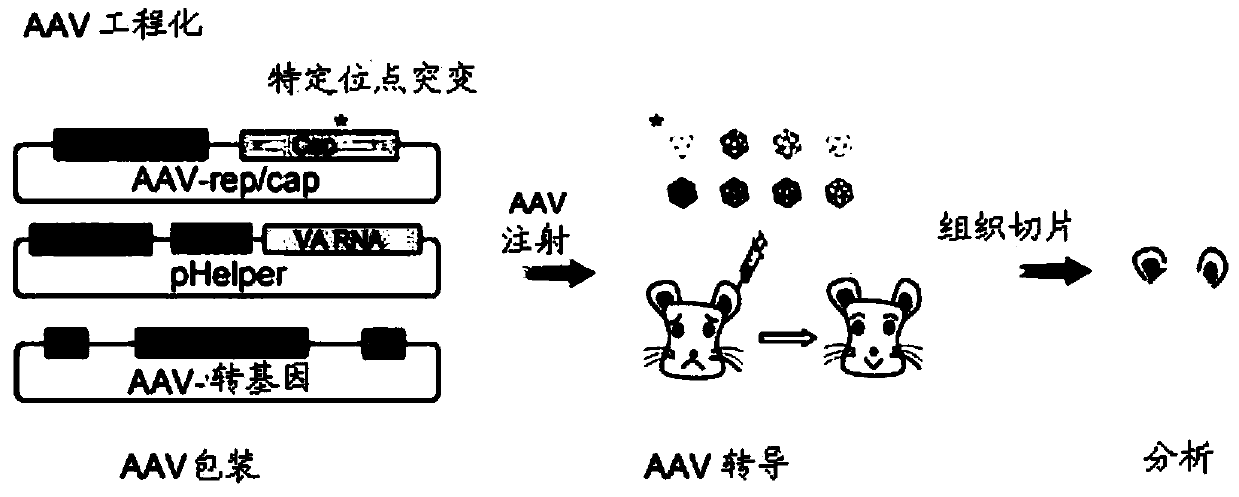
[0137] Example 1: AAV-DJ mutant screening experiment design
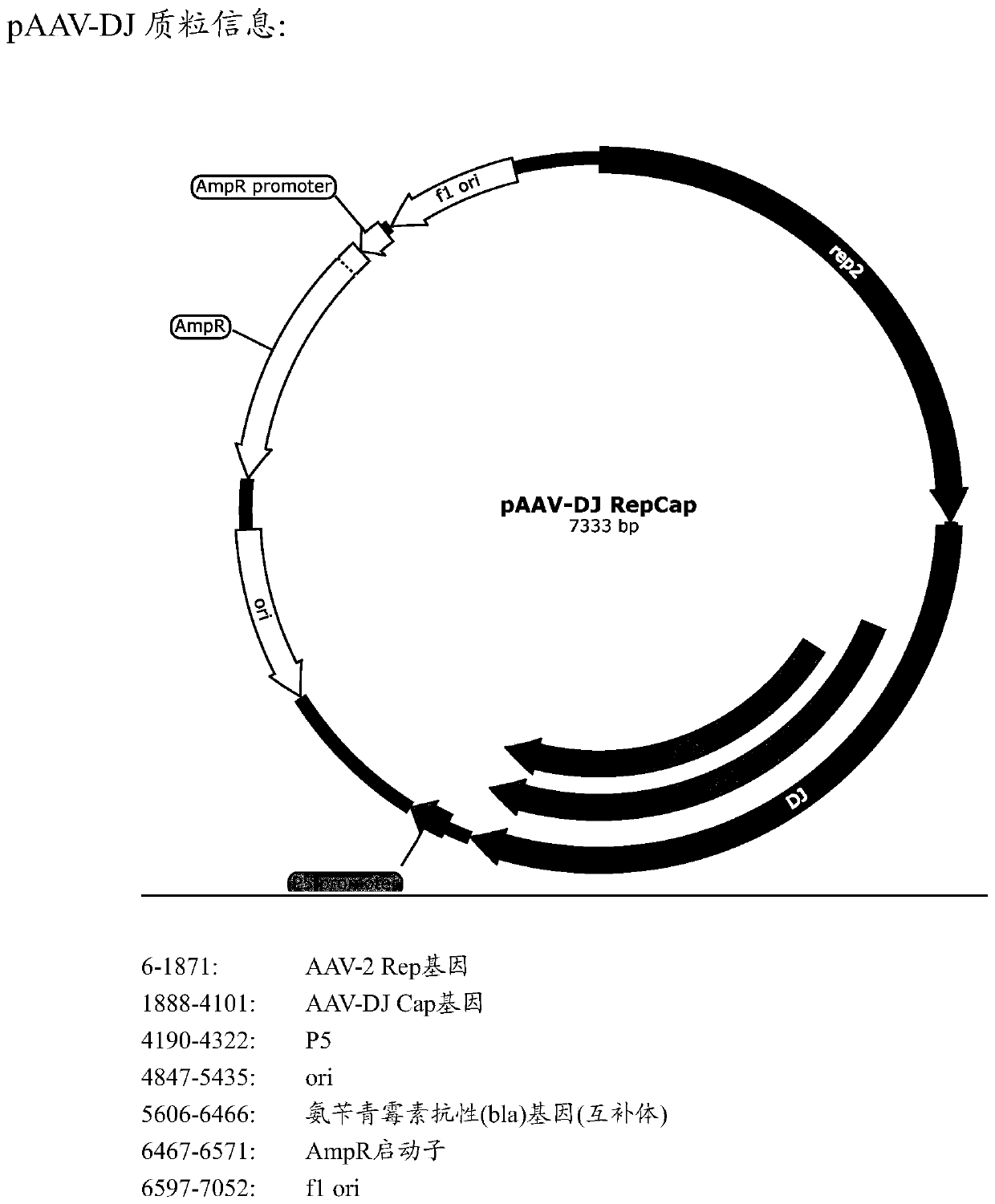
[0138] The cap sequence (SEQ ID No: 1) of AAV-DJ (Institute of Neurology, Chinese Academy of Sciences, see Grimm D et al., J Virol.2008Jun; 82(12):5887-911) was genetically modified to screen for efficient infection support Cell AAV vector. Specifically, the inventors designed three AAV-DJ capsid protein mutants, each containing the following substitutions: S491A, S500A, and S666A, and the corresponding polynucleotides were obtained by PCR amplification (all alanines at the mutation site are Encoded by GCC, the nucleotides at the remaining positions are the same as the AAV-DJ cap gene sequence), and then the corresponding AAV-rep / cap mutant plasmid (see Figure 1B ). The virions of AAV-DJ and its mutants (3 species) were packaged using a three-plasmid system, and then injected into the cochlea of P0 ICR mice. After 2-3 weeks, the materials were taken for fluorescence observation and phenotype of the support cell inf...
Embodiment 2
[0140] Example 2: Infection efficiency in mouse cochlea
[0141] AAV-DJ and its mutant viruses were injected into the cochlea of PO ICR mice. Each mouse is injected 4×10 9 vgAAV virus. Similarly, 3 weeks after the injection, the cochlear basement membrane of the mouse was stripped and stained, and the proportion of Tdtomato positive cells in the supporting cells of the apical, middle and basal parts were counted.
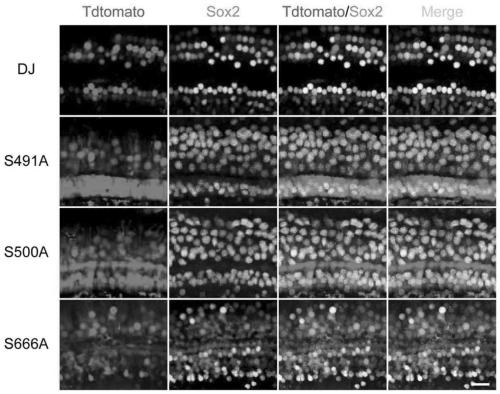
[0142] The experimental results showed that in AAV-DJ mutants (S491A, S500A, and S666A), 55.7%, 51.42% and 45.0% of the supporting cells (sox2 positive) in the top part of the AAV-DJ mutants were Tdtomato positive, which was higher than the 39.6 of AAV-DJ. %, but no significant difference ( Figure 2A , image 3 ).
[0143] In the AAV-DJ mutants (S491A, S500A and S666A), 64.4%, 41.9% and 49.2% of the supporting cells (sox2 positive) in the middle part were Tdtomato positive, which was significantly higher than the 32.0% of AAV-DJ ( Figure 2B , image 3 ).
[0144] In t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com