Method for screening drug target genes based on CRISPR/Cas9 high-throughput technology
A high-throughput drug screening technology, applied in the field of high-throughput sequencing, can solve problems such as skewed distribution of sgRNA libraries, low quality of sgRNA libraries, and a large number of PCR enzymes, so as to save screening costs and increase virus titers , to reduce the effect of false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Screening of drug gene targets for breast cancer cell T47D. The specific method is as follows:
[0037] (1) sgRNA library design:
[0038] Use the websites http: / / crisprscan.org and http: / / www.e-crisp.org to design sgRNAs for gene groups of interest online, and design 10 sgRNAs for each gene.
[0039] (2) Establishment of sgRNA library by electroporation
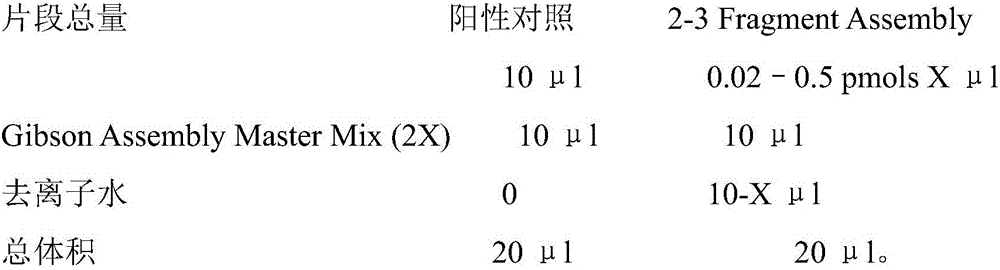
[0040] The oligo fragment of the synthesized sgRNA was amplified by PCR and then ligated into the lentiCRISPRv2 plasmid by Gibson assembly. The ligation product was transformed into a competent state with a Bio-rad electroporator to obtain the sgRNA library.
[0041] (3) Packaging sgRNA library with lentivirus:
[0042] Under sterile conditions, culture 293FT cells, and use Roche's transfection reagent X-tremeGENE HP DNATransfection Reagent to package Lenti CRISPRv2 and two other virus packaging plasmids psPAX2 and pMD2.G into lentivirus. The medium was changed 15 hours after cell transfection, and the virus liqu...
Embodiment 2
[0053] CRISPR / Cas9 applied to the prostate cancer cell line LNcap is applied to a CRISPR / Cas9 screening method for large-scale screening of cancer gene targets, including the following steps:
[0054] (1) sgRNA library establishment:
[0055] Use the websites http: / / crisprscan.org and http: / / www.e-crisp.org to design and genome-wide sgRNA online. The sgRNA carrier uses Lenti CRISPRv2.
[0056] (2) Packaging sgRNA library with lentivirus:
[0057] Under sterile conditions, culture 293FT cells, and use Roche's transfection reagent X-tremeGENE HP DNATransfection Reagent to package Lenti CRISPRv2 and two other virus packaging plasmids psPAX2 and pMD2.G into lentivirus. Transfect 293FT cells according to the ratio of 4:3:1. Taking a 10cm culture dish as an example, the plasmid masses of Lenti CRISPRv2, psPAX2, and pMD2.G are 6μg, 4.5μg, and 1.5μg, respectively, and the amount of X-tremeGENE HP DNA Transfection Reagent 30 μL. The medium was changed 15 hours after cell transfecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com