Human parainfluenza virus type 3 (HPIV-3) wild strain and application thereof
A technology of parainfluenza and wild strains, applied in the direction of viruses, viral peptides, antiviral agents, etc., can solve the problems of drugs and vaccines without specific effects, and achieve the effect of preventing infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Isolation and purification of HPIV-3 wild strain
[0045] 1. Isolation of HPIV-3 wild strain
[0046] Candidate strains must have complete records, history, and sources. Determine the biological characteristics of the candidate virus strains. The attenuated live vaccines and subunit vaccines prepared by the candidate virus strains should have high virus yields, strong ability to induce immune protection, broad cross-protection spectrum, and stable biological characteristics. Virus strains with broad epidemic potential at present and in the future selected by molecular epidemiology and molecular epidemiology.
[0047] The source of the virus: the lower respiratory tract secretions of children hospitalized with lower respiratory tract infection in Maternal and Child Health Hospital of Gansu Province.
[0048]Isolation of virus strains: Add 0.5ml of the obtained secretion into 3ml of DMEM (Dulbecco's Modified Eagle Medium, containing various amino acids and glu...
Embodiment 2
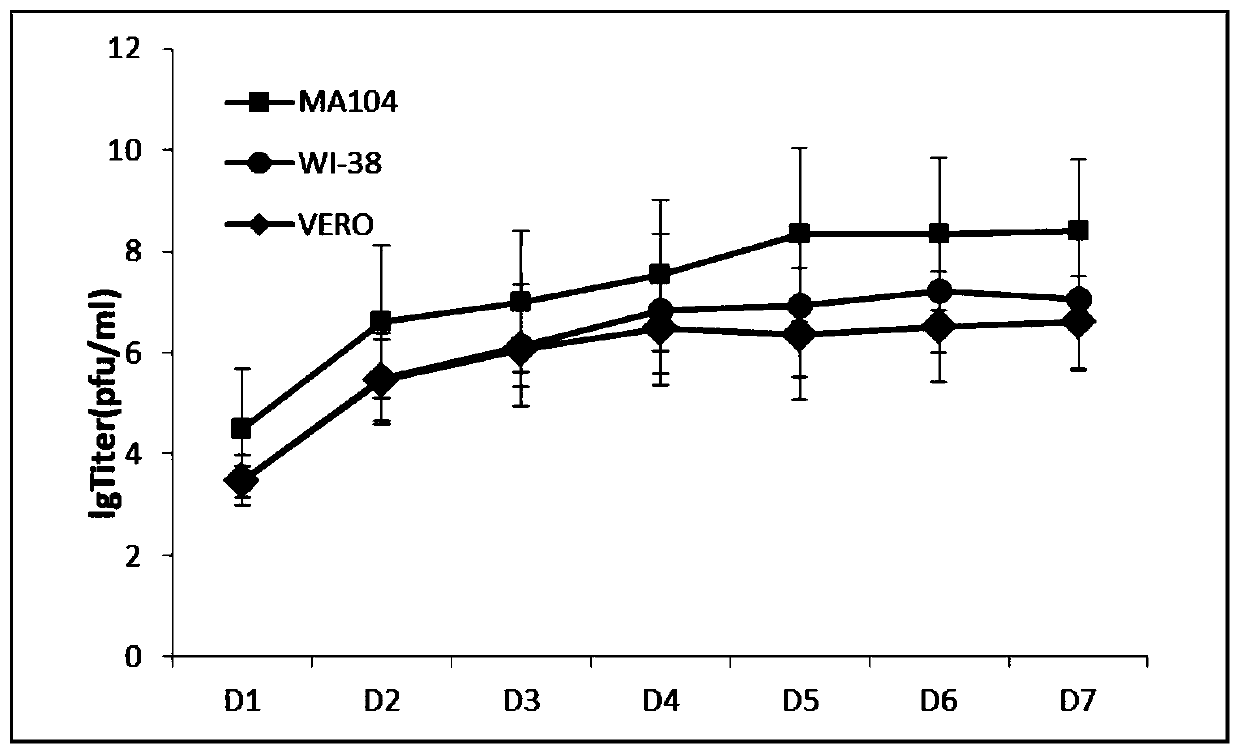
[0067] Example 2 Determination of virus titer
[0068] Determination of virus titer by plaque assay
[0069] HPIV3LZ1728C19 virus was diluted 10x serially, inoculated with MA104 cells in 6-well plates, and placed at 37°C, 5% CO 2 Incubate in an incubator for 2 hours, suck out the supernatant, add 3ml of 0.8% low melting point agarose, after solidification at room temperature, place it upside down at 37°C, 5% CO 2 Cultivate in an incubator for 72 hours, add 3ml of 1% low-melting point agarose containing neutral red, and solidify at room temperature at 37°C, 5% CO 2 Incubate overnight, observe plaques, and take the highest dilution of plaques as the virus titer.
[0070] The highest virus titer obtained by plaque assay was 10 9 pfu / ml, it can be seen that the HPIV3LZ1728C19 virus can obtain a higher virus titer through cell culture.
Embodiment 3
[0071] A large amount of preparation and purification of embodiment 3 HPIV-3 virus culture fluid
[0072] 1. Preparation of HPIV-3 virus culture solution.
[0073] Inoculate the HPIV3LZ1728C19 virus strain, which was cloned and purified to remove specific peripheral factors, into WI-38 cells at 0.05 MOI, at 37°C, 5% CO 2 Culture in an incubator for 7 days, collect the culture supernatant, detect the virus titer, and freeze at -80°C.
[0074] 2. The HPIV-3 virus was purified by ultracentrifugation.
[0075] The HPIV3LZ1728C19 virus culture solution was centrifuged at 8000rpm at 4°C for 30min to precipitate cell debris and collect the supernatant. Take 30ml of the supernatant and add it to a 50ml special centrifuge tube, gently push in 15ml of 50% sucrose from the bottom with an 8cm long needle, pay attention to keep the interface clear, put it into an ultracentrifuge (model: Hitachi CP 70MX) for 4 Centrifuge at 35,000 rpm for 4 hours, discard the supernatant; resuspend the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com