Single-tube multiplex fluorescence PCR (Polymerase Chain Reaction) detection method and kit for 1,2,3-type parainfluenza viruses
A detection method and multiple fluorescence technology, applied in the field of molecular biology detection, can solve the problems of time-consuming and labor-consuming, cumbersome operation, and inability to detect human parainfluenza virus at the same time, and achieve the effect of simple and convenient operation, high sensitivity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: the specific experiment of primer, probe
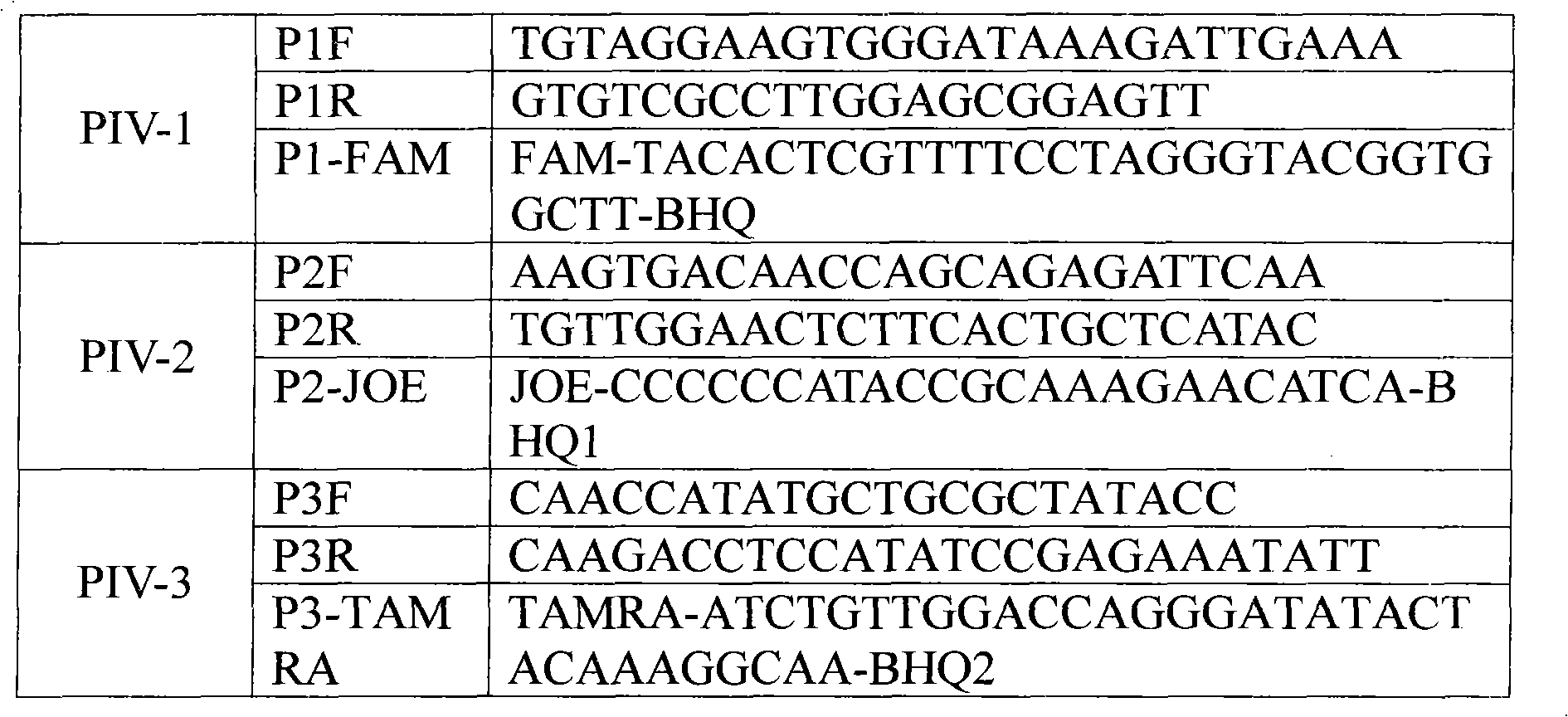
[0060] After using bioinformatics and biological software for designing primers and probes to compare and analyze the sequence data of human parainfluenza virus in the existing database, the primers and probes shown in Table 1 were designed to achieve type 1, 2, and 3 One tube of multiplex fluorescent PCR detection of human parainfluenza virus. Table 1 shows the sequences of primers and probes for human parainfluenza virus types 1, 2, and 3.
[0061] Table 1
[0062]
[0063] In order to verify the specificity of the designed primers and probes, a single-plex fluorescent PCR reaction was designed first, and the primers and probes were evaluated, that is, the three sets of primers and probes of PIV-1, PIV-2 and PIV-3 were detected separately. specificity. The experiment was carried out with positive strains isolated from culture, and the positive strains came from Guangzhou Institute of Respiratory Diseases a...
Embodiment 2
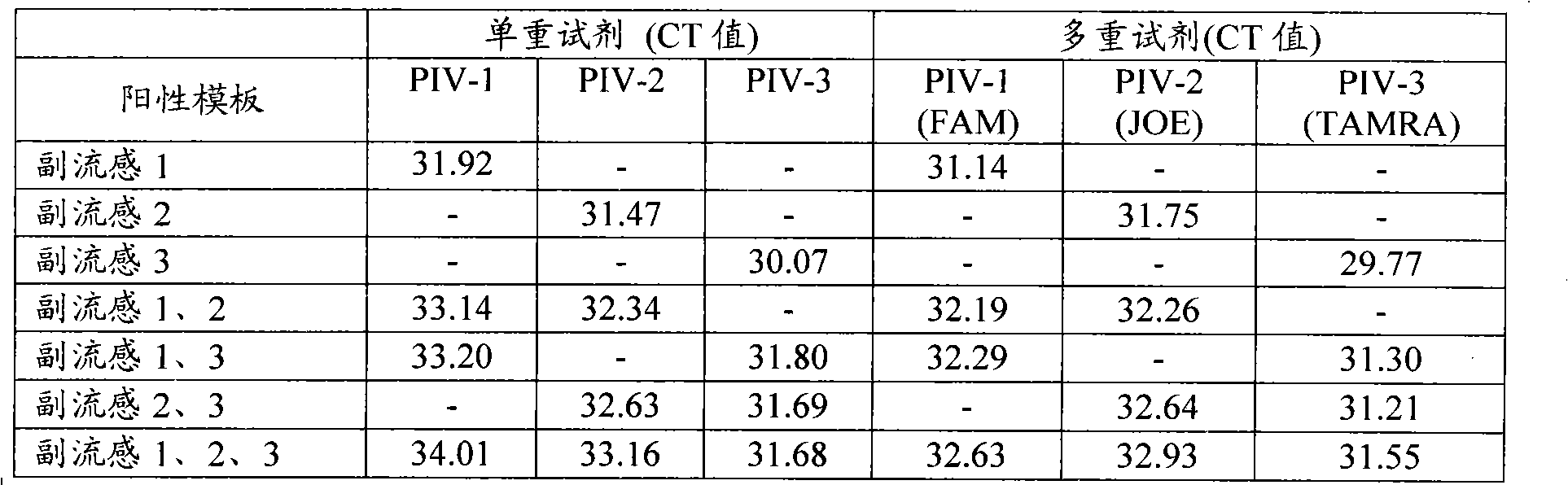
[0069] Embodiment 2: the specificity experiment of PIV multiple detection reagent
[0070] The multiple detection reagents are prepared using the RT-PCR buffer in TAKARA'PrimeScript One Step RT-PCR Kit (Ver.2)' (TAKARA'PrimeScript One Step RT-PCR Kit Ver.2'), and the reagents in Table 1 The primers of PIV-1, 2, and 3 and three kinds of probes were prepared into a tube of reaction solution (M-PIV123). The volume of the prepared reagents was 18 μl / reaction. 0.5-5 pmol; reserve 2 μl for adding enzyme mixture, reserve 5 μl for adding template; total reaction volume is 25 μl / reaction.
[0071] Use single positive template (parainfluenza 1, parainfluenza 2 or parainfluenza 3); double positive template (parainfluenza 1 and 2 mixed; parainfluenza 1 and 3 mixed or parainfluenza 2 and 3 mixed); triple positive template (parainfluenza 2 and 3 mixed); Influenza 1, 2, and 3 mixed) experiments were performed on the multiplex detection reagents, while comparisons were performed using the si...
Embodiment 3
[0075] Embodiment 3: the sensitivity experiment of PIV multiple detection reagent
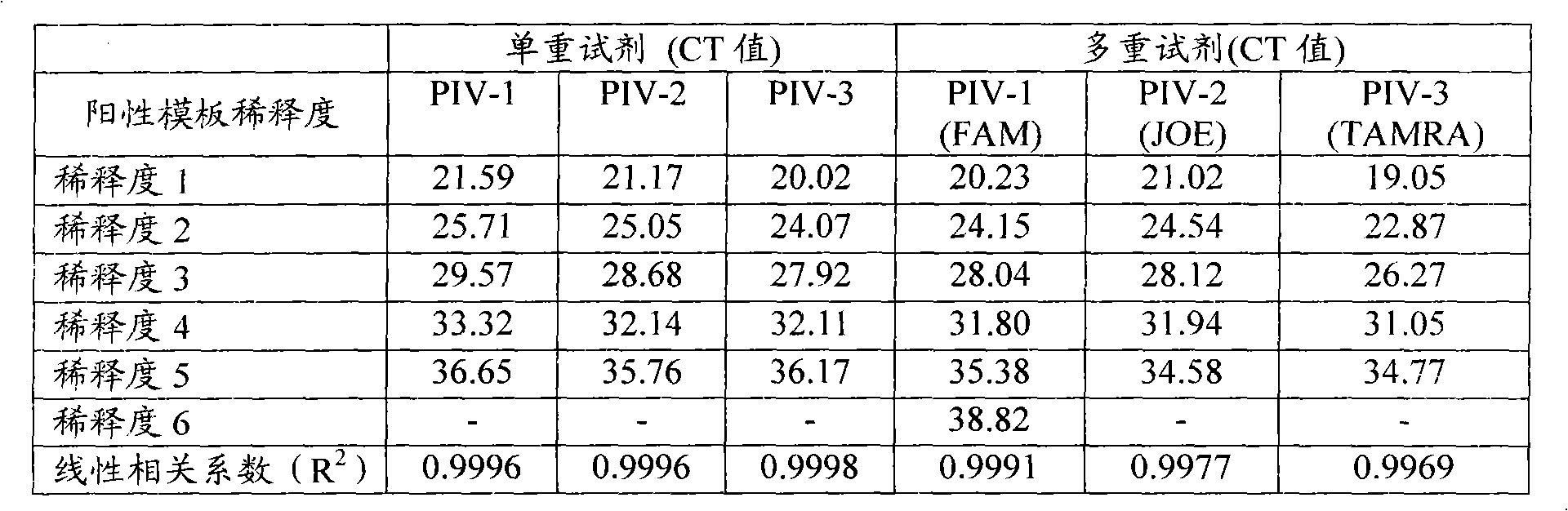
[0076] In order to further test the sensitivity of the reagent, a 10-fold gradient dilution was performed on the positive samples of parainfluenza 1, 2, and 3, and the dilution was 1 to 6, which respectively indicated that the PIV-1, PIV-2, and PIV-3 viruses were diluted 10 to 106 times , were compared with multiple parainfluenza virus detection reagents and single detection, and virus culture detection was carried out at the same time. The results of quantitative PCR experiments are shown in Table 4. The identifiable range of virus culture was between dilution 3 and dilution 4, and the sensitivity was much lower than singleplex or multiplex PIV quantitative PCR detection (data not shown). Table 4 is a data table of comparative experimental results of multiplex and singlex parainfluenza quantitative PCR detection reagents on 10-fold serially diluted viruses.
[0077] Table 4
[0078]
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com