Antibody producing non-human mammals
a technology for antibody production and mammals, applied in the field of production and use of non-human animals, can solve the problems of reducing the efficacy of subsequent administrations, and achieve the effects of reducing the number of administrations, facilitating the opening and maintenance of euchromatin, and preventing silencing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Human Light Chain V-Gene Clones
[0107]This example describes the rationale behind the choice of two human light chain V-genes, one gene of the kappa type and one gene of the lambda type, that are used as a proof of concept for light chain expressing transgenic mice. De Wildt et al. 1999 (de Wildt et al. (1999), J. Mol. Biol. 285(3):895) analyzed the expression of human light chains in peripheral IgG-positive B-cells. Based on these data, IGKV1-39 (O12) and IGLV2-14 (2a2) were chosen as light chains as they were well represented in the B-cell repertoire. The J-segment sequence of the light chains has been chosen based upon sequences as presented in GenBank ABA26122 for IGKV1-39 (B. J. Rabquer, S. L. Smithson, A. K. Shriner and M. A. J. Westerink) and GenBank AAF20450 for IGLV2-14 (O. Ignatovich, I. M. Tomlinson, A. V. Popov, M. Bruggemann and G. J. Winter, J. Mol. Biol. 294 (2):457-465 (1999)).
[0108]All framework segments are converted into germline amino acid sequences to provide the...
example 2
Obtaining Mouse Heavy Chain V-Genes that Pair with Human IGKV1-39 Gene Segment to Form Functional Antibody Binding Sites
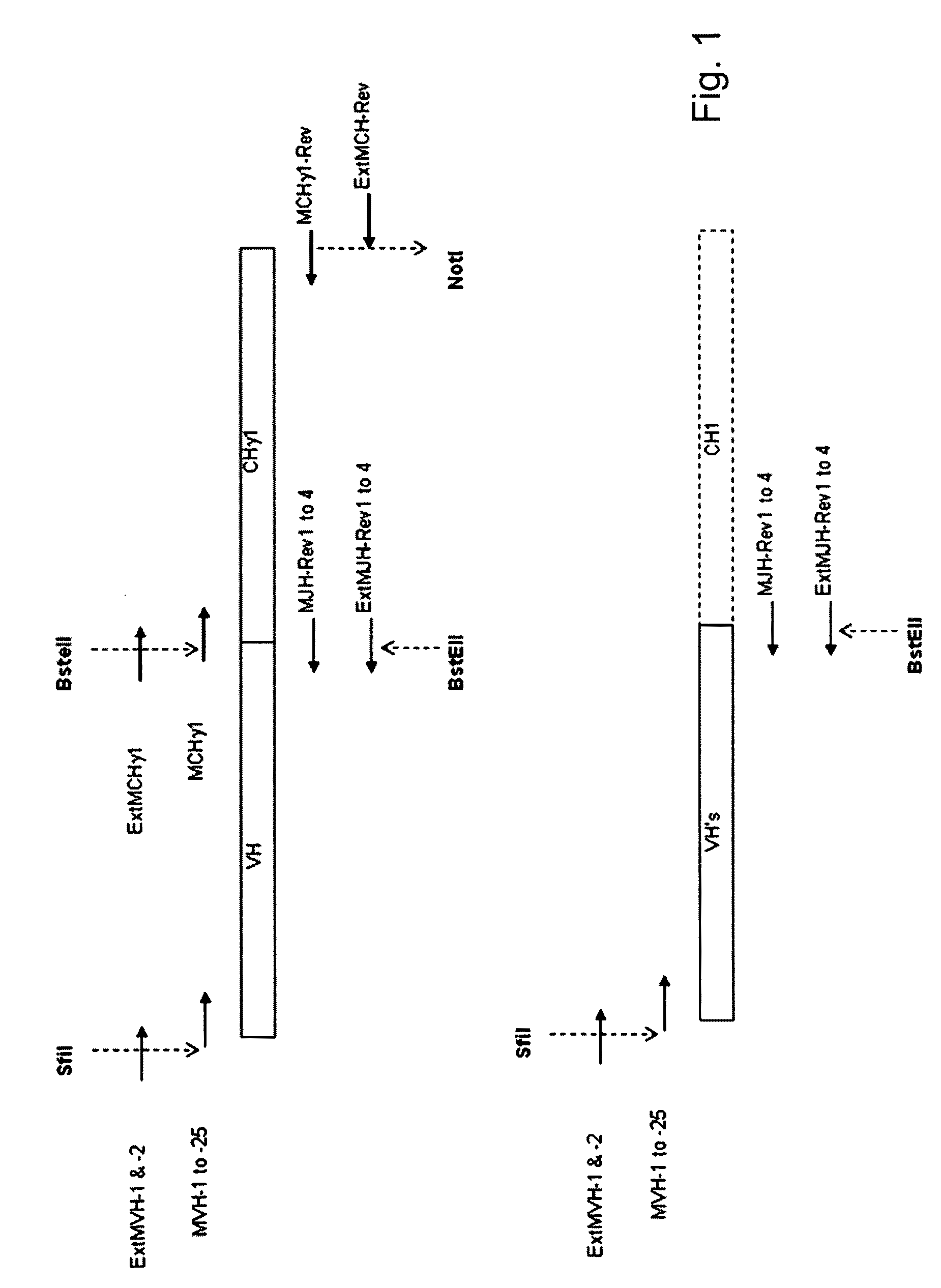
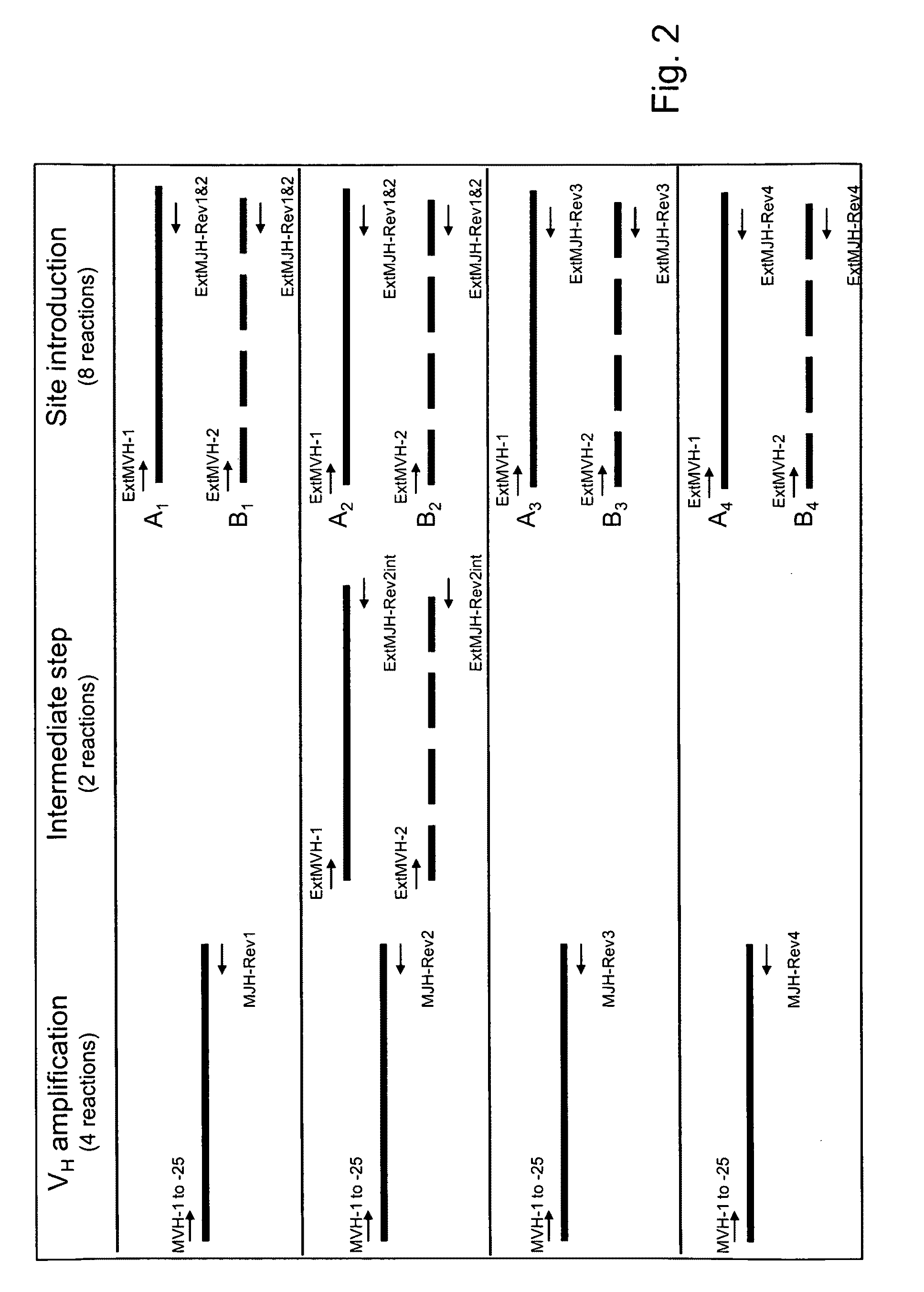
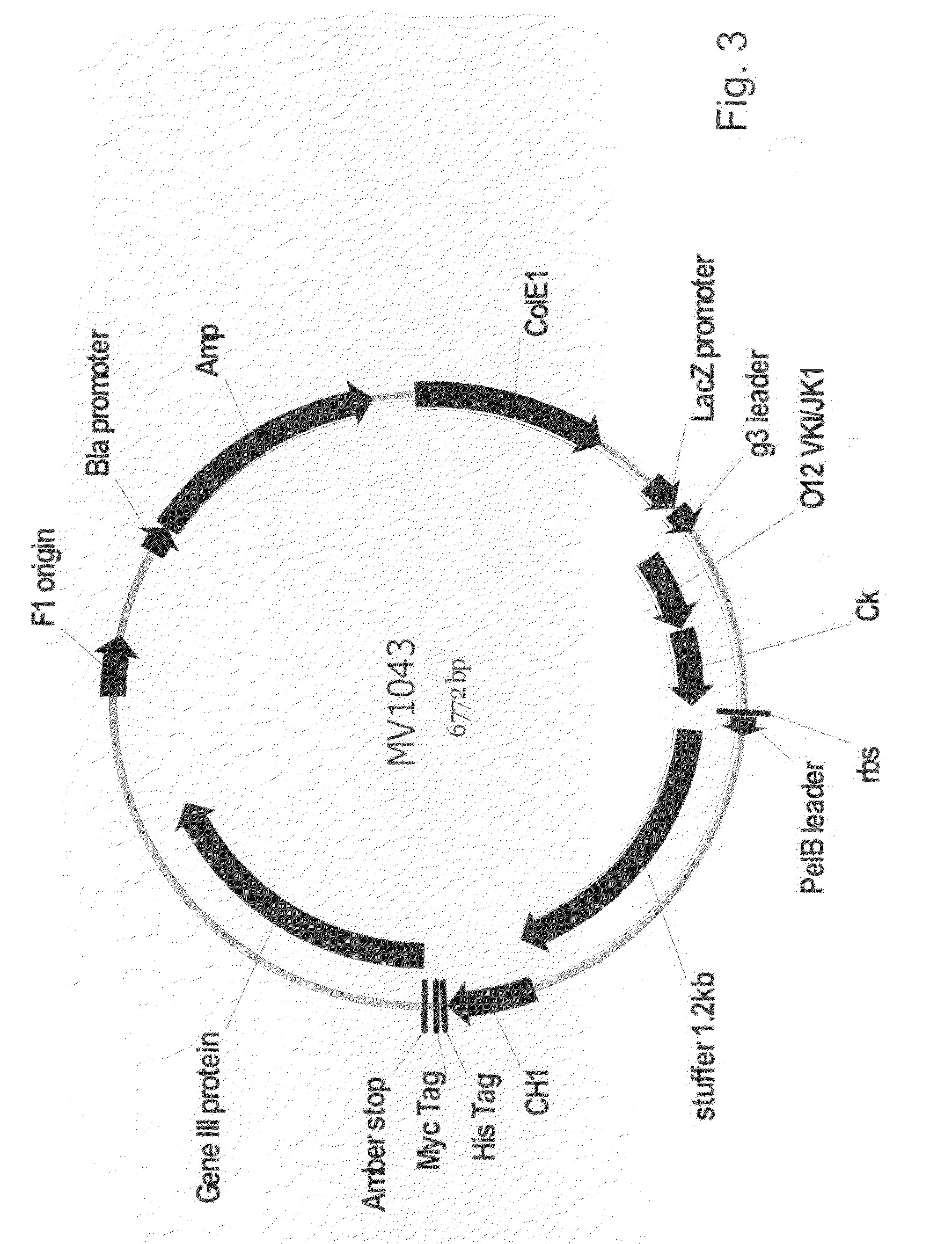
[0109]This example describes the identification of mouse heavy chain V-genes that are capable of pairing with a single, rearranged human germline IGKV1-39 / J region. A spleen VH repertoire from mice that were immunized with tetanus toxoid was cloned in a phage display Fab vector with a single human IGKV1-39-C kappa light chain and subjected to panning against tetanus toxoid. Clones obtained after a single round of panning were analyzed for their binding specificity. The murine VH genes encoding tetanus toxoid-specific Fab fragments were subjected to sequence analysis to identify unique clones and assign VH, DH and JH utilization.
[0110]Many of the protocols described here are standard protocols for the construction of phage display libraries and the panning of phages for binding to an antigen of interest and described in Antibody Phage Display: Methods and Protocols ...
example 3
Silencing of the Mouse Kappa Light Chain Locus
[0125]This example describes the silencing of the mouse endogenous kappa light chain locus. The endogenous kappa locus is modified by homologous recombination in ES cells, followed by the introduction of genetically modified ES cells in mouse embryos to obtain genetically adapted offspring.
[0126]A vector that contains an assembled nucleotide sequence consisting of a part comprising the J-region to 338 by downstream of the J5 gene segment fused to a sequence ending 3′ of the 3′ CK enhancer is used for homologous recombination in ES cells. The assembled sequence is used to delete a genomic DNA fragment spanning from 3′ of the JK region to just 3′ of the 3′ CK enhancer. As a consequence of this procedure, the CK constant gene, the 3′ enhancer and some intergenic regions are removed (see FIGS. 4 and 18).
Construction of the Targeting Vector
[0127]A vector that received 4.5-8 kb flanking arms on the 3′ and 5′ end fused to the deletion segment w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volumes | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com