Respiratory pathogen multi-detection reagent kit
A multiple detection and respiratory technology, applied in the direction of recombinant DNA technology, microbial measurement/inspection, biochemical equipment and methods, etc., can solve the problems of cumbersome operation and long operation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Embodiment 1 A kind of kit for detecting sixteen kinds of respiratory pathogens
[0156] Sixteen respiratory pathogens including 12 RNA viruses (influenza A virus, influenza B virus, influenza A H1N1 virus, respiratory syncytial virus types A and B, parainfluenza virus-1 / -2 / -3, coronavirus OC43, coronavirus 229E, rhinovirus and human metapneumovirus), 2 DNA viruses (adenovirus and bocavirus), and 2 bacteria (Mycoplasma pneumoniae and Bordetella pertussis).
[0157] The kit includes
[0158] (1) Amplification primer set, including the primers shown in the table below, used to pre-amplify the corresponding pathogen target DNA fragment
[0159]
[0160]
[0161] (2) Specific primer set, including the primers shown in the table below, used to make the target DNA fragment corresponding to the pathogen carry specific probe detection sequence and internal fluorescent label
[0162]
[0163]
[0164]
[0165]
[0166] (3) Detection probe set, including the d...
Embodiment 2
[0171] Embodiment 2 A method for detecting sixteen kinds of respiratory pathogens
[0172] The above sixteen respiratory pathogens include 12 RNA viruses (influenza A virus, influenza B virus, influenza A H1N1 virus, respiratory syncytial virus A and B, parainfluenza virus-1 / -2 / -3 , coronavirus OC43, coronavirus 229E, rhinovirus and human metapneumovirus), 2 DNA viruses (adenovirus and bocavirus) and 2 bacteria (Mycoplasma pneumoniae and Bordetella pertussis).
[0173] The detection kit used is the kit described in Example 1 of the present invention.
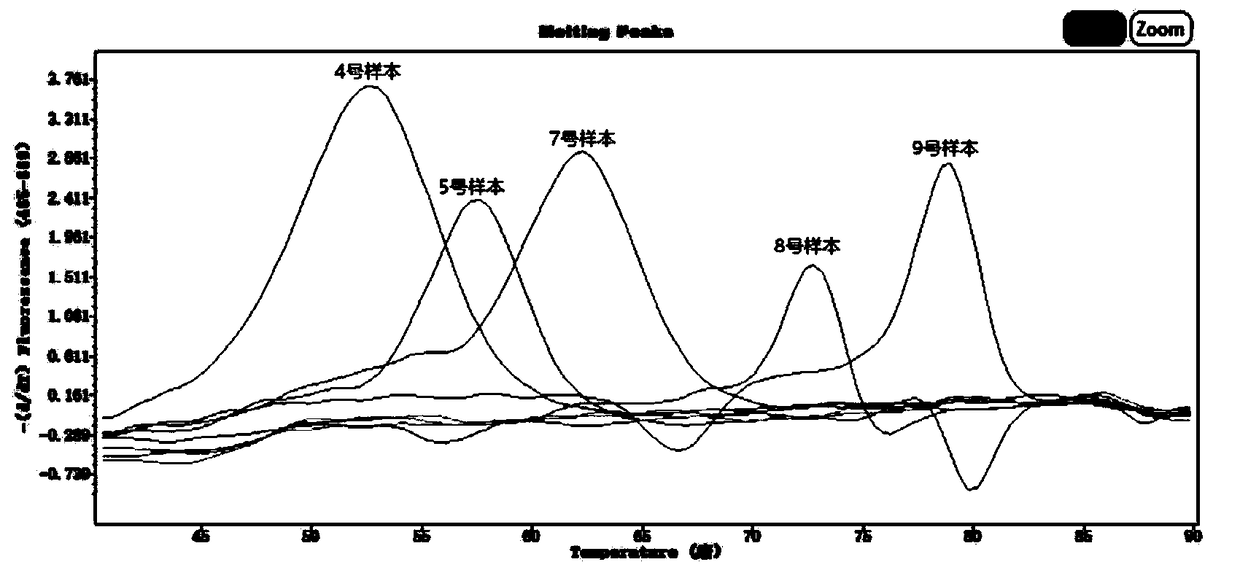
[0174] Samples to be tested: 9 tissue samples from different sources that are known to be infected with respiratory pathogens (within the above-mentioned 16 respiratory pathogens), and the sample numbers are 1-9.
[0175] Detection method:
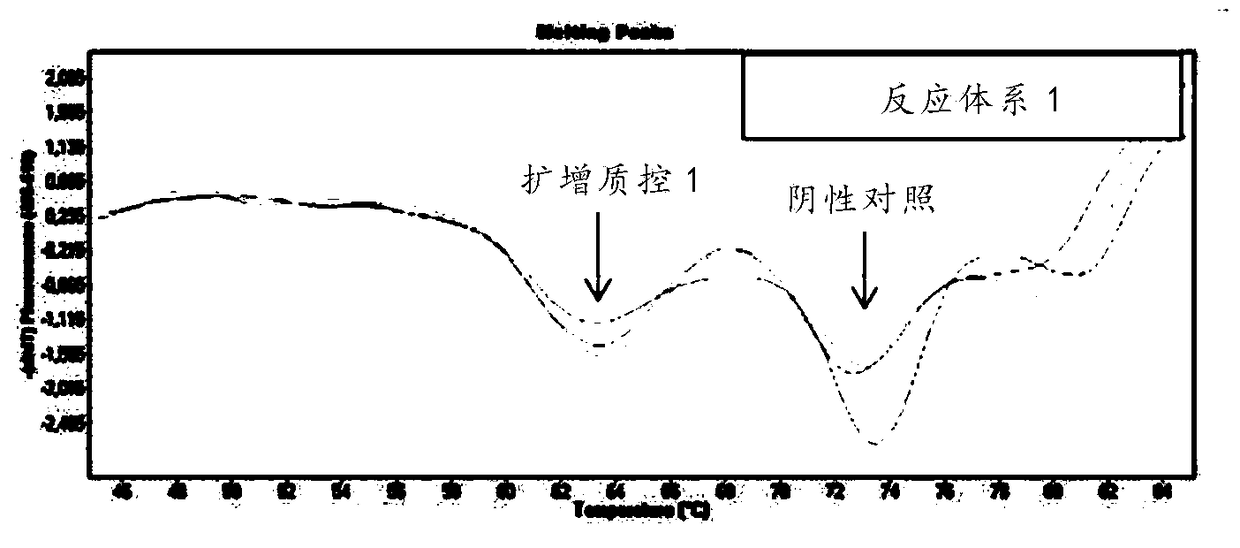
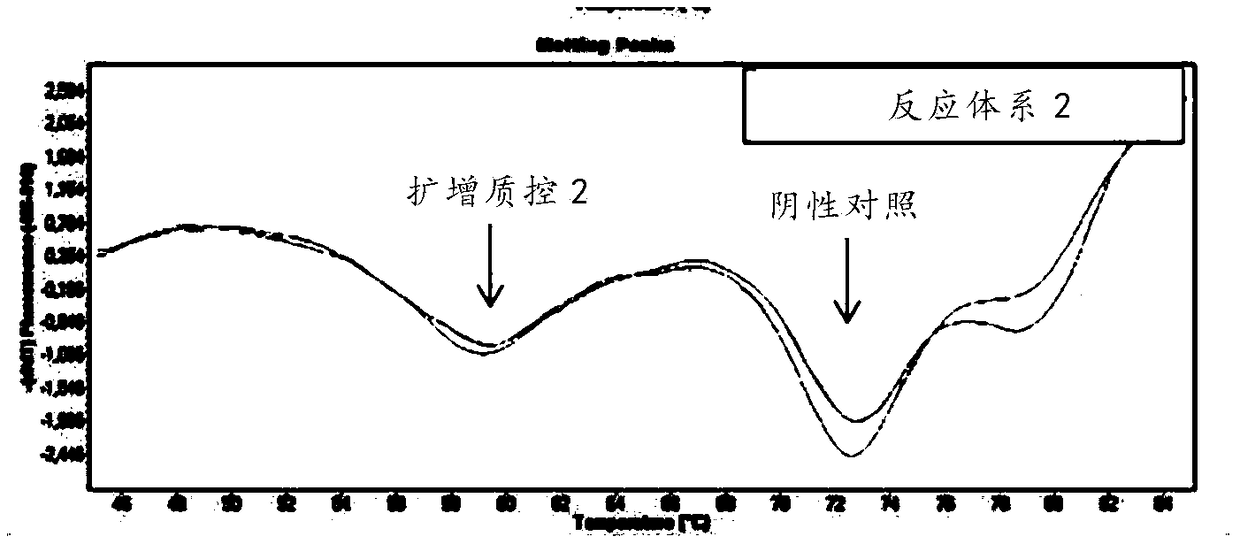
[0176] (1) Sample processing: add 5 μl of internal quality control substance for every 200 μl sample, and then extract nucleic acid;
[0177] (2) Negative control: 200 μl preservation sol...
Embodiment 3
[0202] Embodiment 3 A method for detecting sixteen kinds of respiratory pathogens
[0203] The detection kit used is the kit described in Example 1 of the present invention;
[0204] Samples to be tested: Sixteen kinds of pathogenic whole viruses or whole bacterial nucleic acids respectively stored in the sample preservation solution, one of which was taken, and 5 samples were prepared by gradient dilution;
[0205] The above-mentioned 5 samples were detected according to the detection method in Example 2, and 16 kinds of pathogens were detected sequentially. According to the detection limit of the corresponding pathogen when the positive rate was greater than or equal to 95%, by using the QX200TMDroplet Digital of Bio-Rad Company The absolute copy number obtained by TMPCR is used to calculate the detection limit), and the following table shows the detection limits of the sixteen kinds of pathogens tested:
[0206] Pathogen
[0207] It can be seen from the detection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com