Libaweilin slow-released pill
A technology for sustained-release pellets and ribavirin, which is applied in the directions of drug delivery, organic active ingredients, and medical preparations containing active ingredients, etc. The effect of prolonging plasma elimination half-life and improving antiviral efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 The preparation of ribavirin sustained-release pellets and sustained-release granules:
[0025] Formula Each bag of granules contains 0.25g of ribavirin
[0026] 1. Prescription with pill core
[0027] Ribavirin (120 mesh) 300g
[0028] Microcrystalline cellulose (120 mesh) 280g
[0029] Pill core (40-60 mesh) 280g
[0030] 3% hypromellose appropriate amount
[0031] Make 1000 bags
[0032] 2. Prescription of coating solution: dosage per 500g pill core
[0033] Eudragit NE 30D 156.7g
[0034] Talc powder (1250 mesh) 48.57g
[0035] Sodium lauryl sulfate 0.392g
[0036] Water 186.5ml Preparation process 1. Mold release
[0037] Weigh the microcrystalline cellulose and put it in the centrifugal coating granulator to start the centrifugal coating granulator according to the following parameters. The speed of the main engine is 200rpm, the blast flow rate is 20×20L / min, the jet flow rate is 15-20L / min, the jet pressure is 0.5Mpa, and the spray pump sp...
Embodiment 2
[0041] Embodiment 2 release inspection
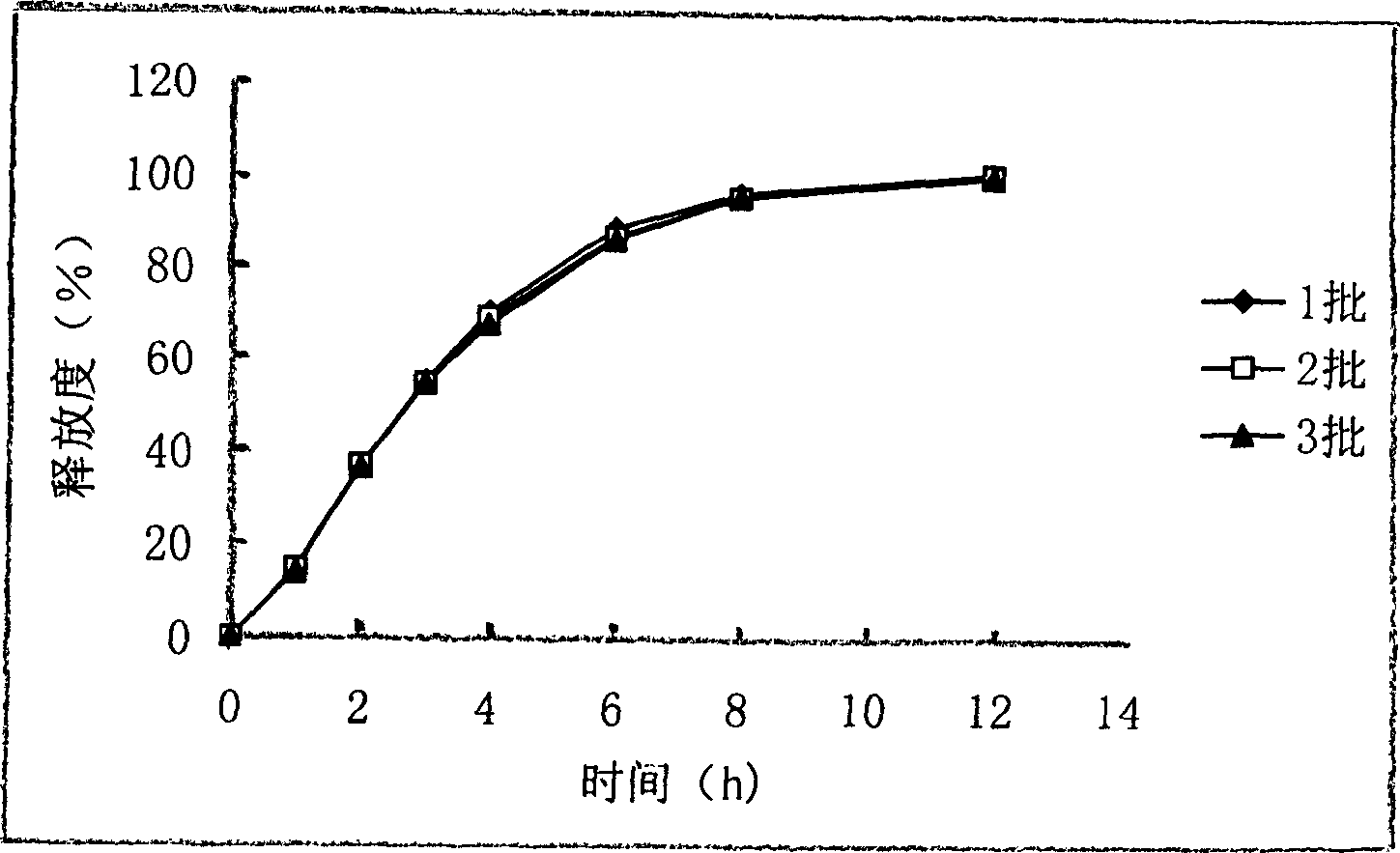
[0042] The granules prepared in Example 1 were tested for the release of the granules prepared in Example 1 with reference to the method for measuring the release of other varieties recorded in the second part of the Chinese Pharmacopoeia 2000 edition, and the medium was water. The sampling time points were determined as 2 hours, 4 hours, and 8 hours. Its release curve is shown in figure 1. Example 3 Preparation of Ribavirin Sustained-release Pellets and Sustained-release Capsules Prescription of drug-containing pill core: Ribavirin (120 mesh) 250g microcrystalline cellulose (120 mesh) 7.5g pellet core (32-40 mesh) Appropriate amount of 3% hydroxypropyl methylcellulose is made into 1000 capsules 2 Coating solution Prescription: dosage per 500g of drug-containing pellets Eudragit NE 30D 83.33g talcum powder (1250 mesh) 25.83g sodium lauryl sulfate 0.21g water 99.16 ml Preparation process 1. The operation of the mold drawing process is...
Embodiment 4
[0043] Embodiment 4 release degree measurement
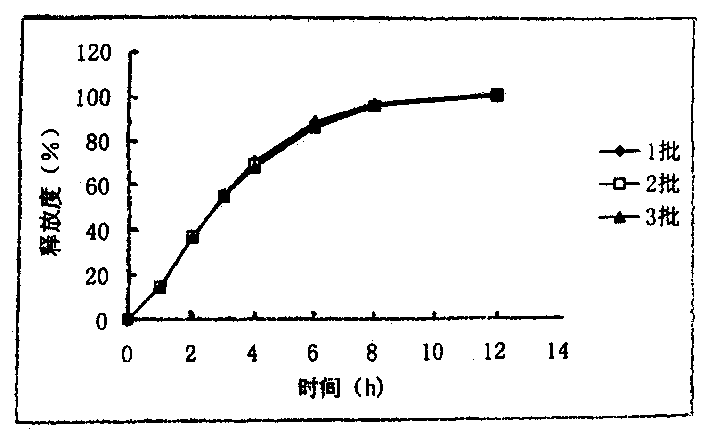
[0044] Get the capsule prepared by the method of Example 3, according to the dissolution method (the second method of appendix XC of the Chinese Pharmacopoeia version in 2000), 900ml of water is used as solvent, and the rotating speed is 50 revolutions per minute, according to the law. The degree of release at 2, 6, 8 hours. Its release curve is shown in figure 2 . Description of drawings: figure 1 is the release curve of ribavirin sustained-release granules,
[0045] figure 2 is the release profile of ribavirin sustained-release capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com