Mesoporous silicon dioxide-methotrexate-mitoxantrone nanoparticles as well as preparation, activity and application thereof
A technology of mesoporous silica and methotrexate, applied in mesoporous silica-methotrexate/mitoxantrone nanoparticles, MSNN-MTX/MIT can solve the problem in the field of preparing MTX and MIT There is no problem of improving the survival period, cure rate and toxicity of cancer patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Preparation of MSNN-MTX / MIT
[0018] 1) Preparation of MSNN
[0019] Dissolve 1 g of cetyltrimethylammonium bromide in 480 mL of distilled water, add 3.5 mL of aqueous sodium hydroxide solution (2M) with stirring, heat at 80°C for 1 h, and stir vigorously until the solution becomes clear. Then, 5 mL of tetraethyl orthosilicate and 1 mL of 3-aminopropyltrimethoxysilane were added rapidly, and heated at 80° C. for 2 h. The reaction mixture was cooled, filtered, and the filter cake was washed three times with 20 mL of distilled water and three times with 20 mL of ethanol, and dried under vacuum at 70°C for 24 hours. The dried solid was refluxed in a mixed solvent of 350mL methanol and 7mL concentrated hydrochloric acid for 24h. The reaction mixture was cooled, filtered, and the filter cake was washed three times with 20 mL of distilled water and three times with 20 mL of ethanol, and dried under vacuum at 70°C for 24 hours. 1.2 g of amino-functionalized mesopo...
Embodiment 2
[0025] Example 2 Determination of the nanostructure of MSNN-MTX / MIT
[0026] 1) Determination of scanning electron microscope Weigh 1 mg of MSNN, MSNN-MTX and MSNN-MTX / MIT respectively, disperse it with 1.5 mL of absolute ethanol ultrasonically, take 100 μL and drop it on the surface of a glass slide, dry it at 37°C for 3 days, and place it on a scanning electron microscope (JSM -6360LV, JEOL, Japan) to observe its shape, see the results Figure 5 .
[0027] 2) Determination by transmission electron microscope Weigh 1 mg of MSNN, MSNN-MTX and MSNN-MTX / MIT respectively, disperse them with 1.5 mL of absolute ethanol ultrasonically, take about 10 μL and drop them on the surface of the copper grid of the transmission electron microscope, dry them at 37°C for 3 days, and put them on the surface of the transmission electron microscope. (JEM-1230, JEOL, Japan) to observe its morphology, see the results Figure 5 .
[0028] 3) Determination of Zeta Potential Weigh 1 mg MSNN, MSNN-M...
Embodiment 3
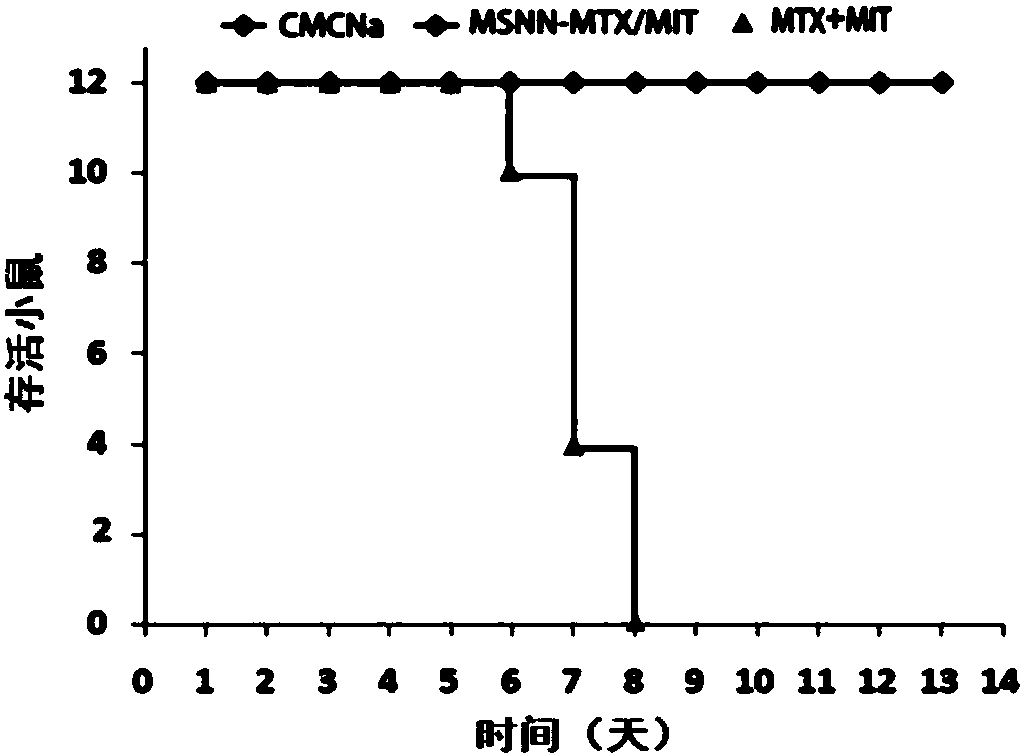
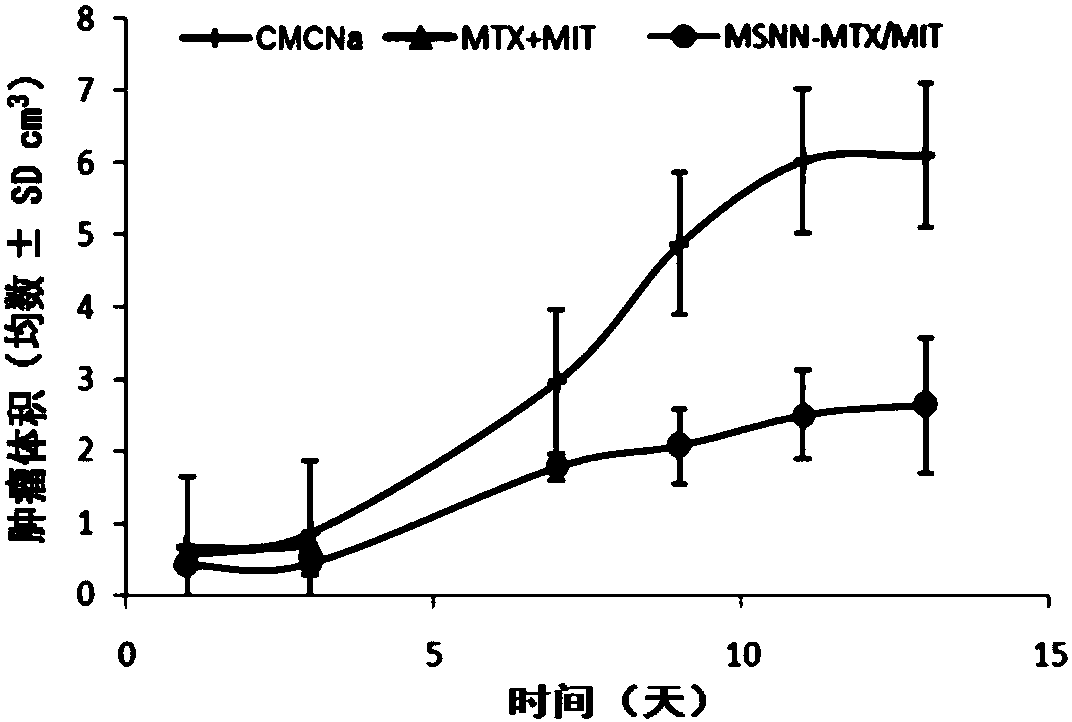
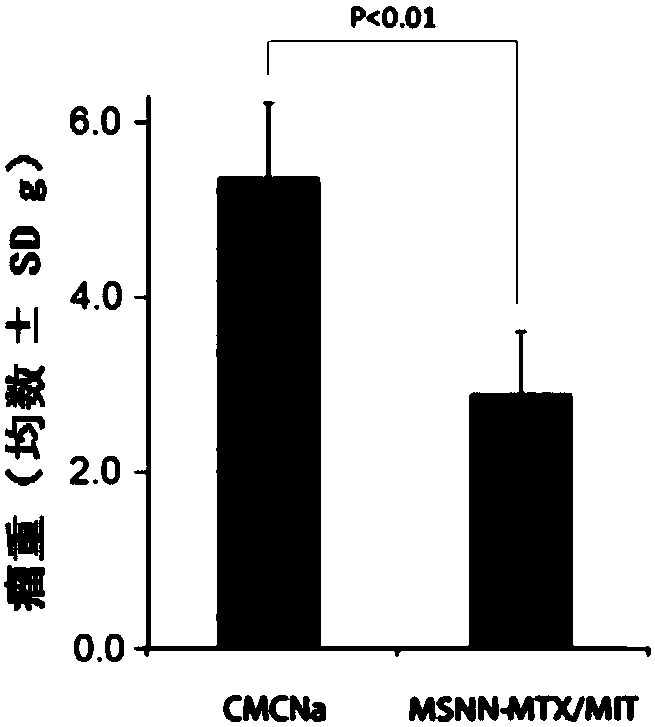
[0029] Embodiment 3 evaluates the antitumor activity of MSNN-MTX / MIT, mouse survival period and toxicity
[0030] For male ICR mice (weight 20±2g, purchased from Beijing Weitong Lihua Animal Experiment Technology Co., Ltd.), the S180 tumor fluid was diluted to 1.5×10 7 Each mouse was inoculated with 0.2 mL of tumor fluid in the right armpit, and the tumor volume was measured with a vernier caliper every day to observe the tumor growth. The tumor volume grows to 1.0cm within 7 days after inoculation of tumor fluid 3 At , all mice were weighed and randomly divided into 3 groups, 12 mice in each group, receiving CMC-Na treatment, MTX+MIT treatment and MSNN-MTX / MIT treatment respectively. Pass MSNN-MTX / MIT through a 200-mesh sieve, use physiological saline containing 5‰ CMC-Na as a vehicle, and administer it by intraperitoneal injection. The dosage of CMC-Na group was 0.2mL per mouse, the dosage of MTX+MIT group was 4mg / kg MTX and 0.5mg / kg MIT, and the dosage of MSNN-MTX / MIT gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com