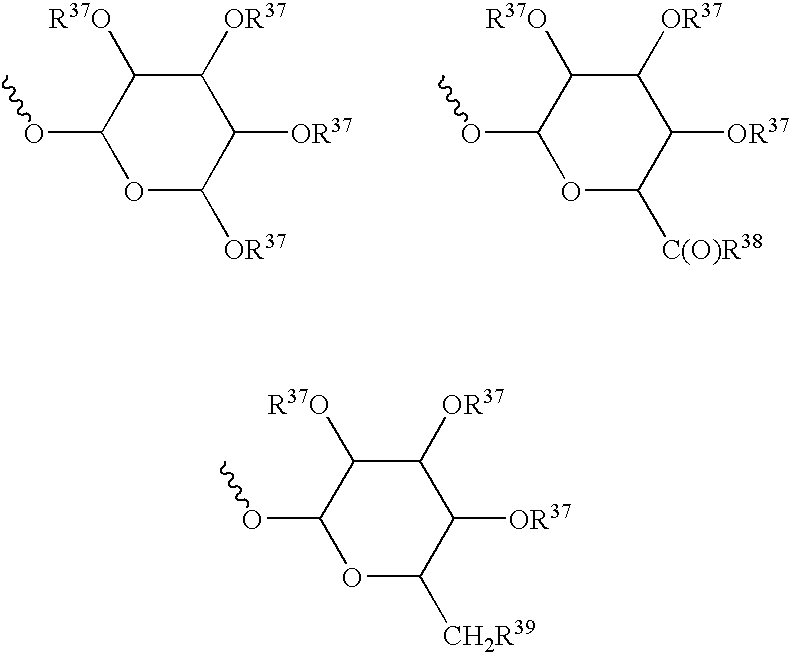
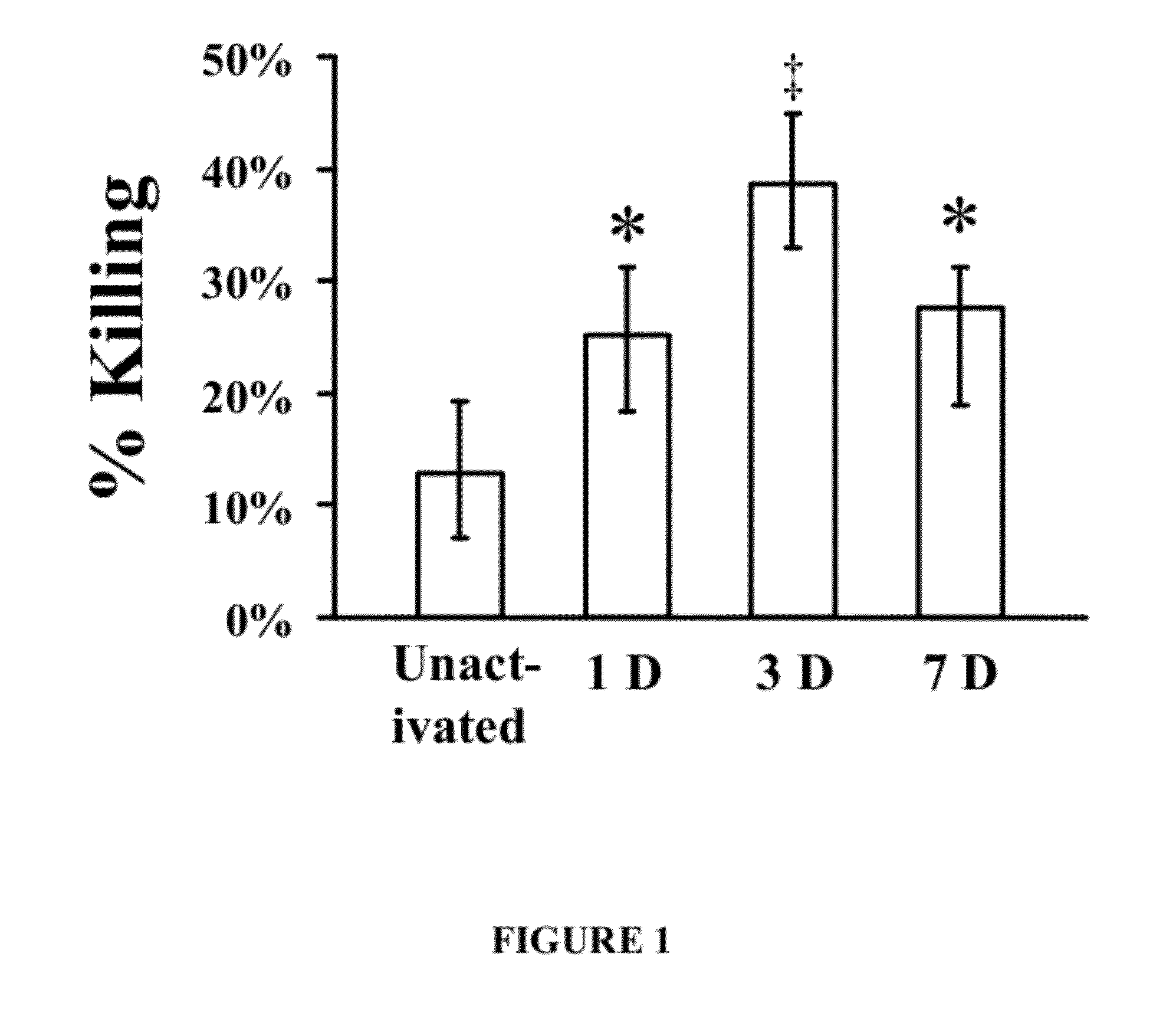
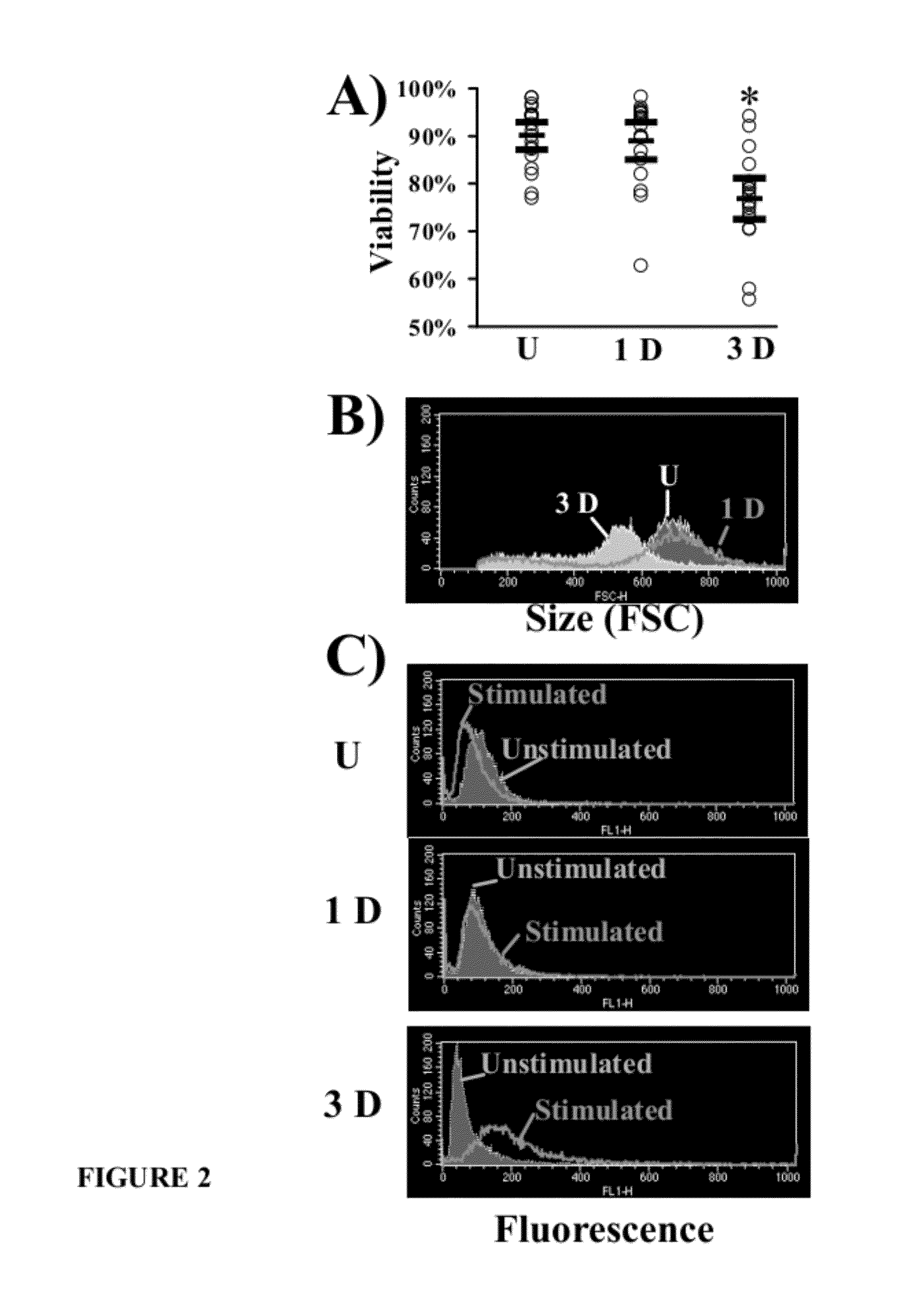
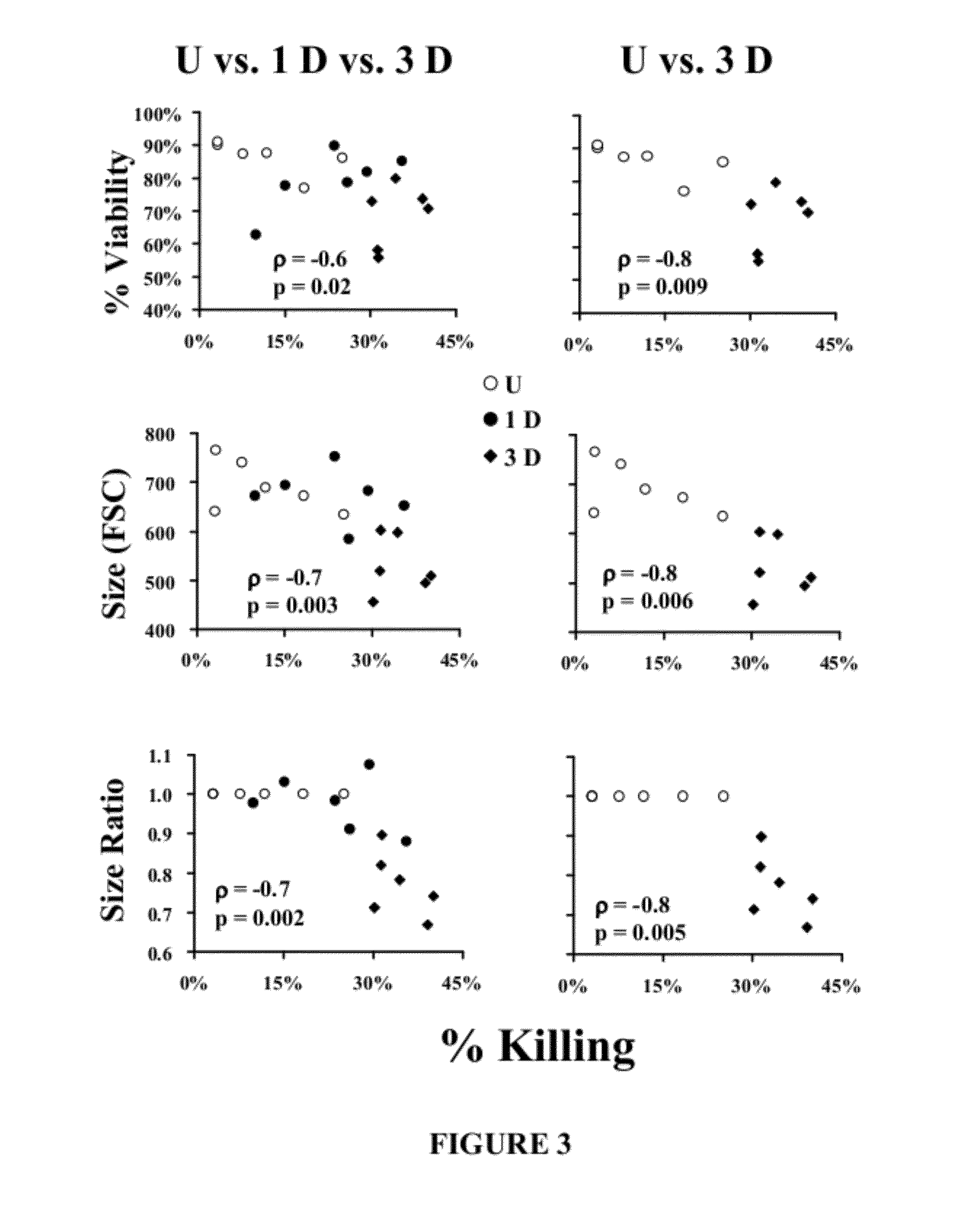
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Granulocytopenias" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
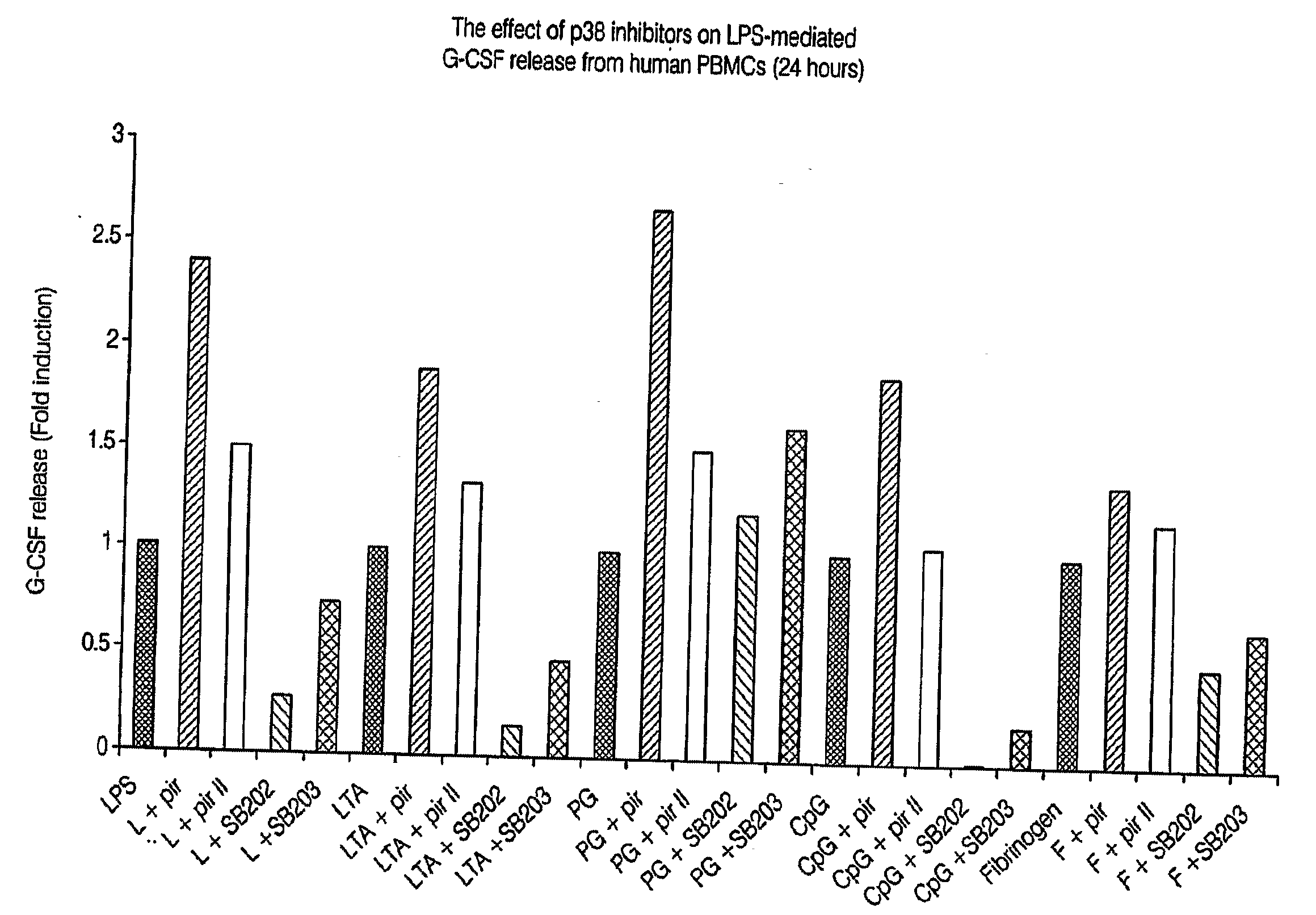
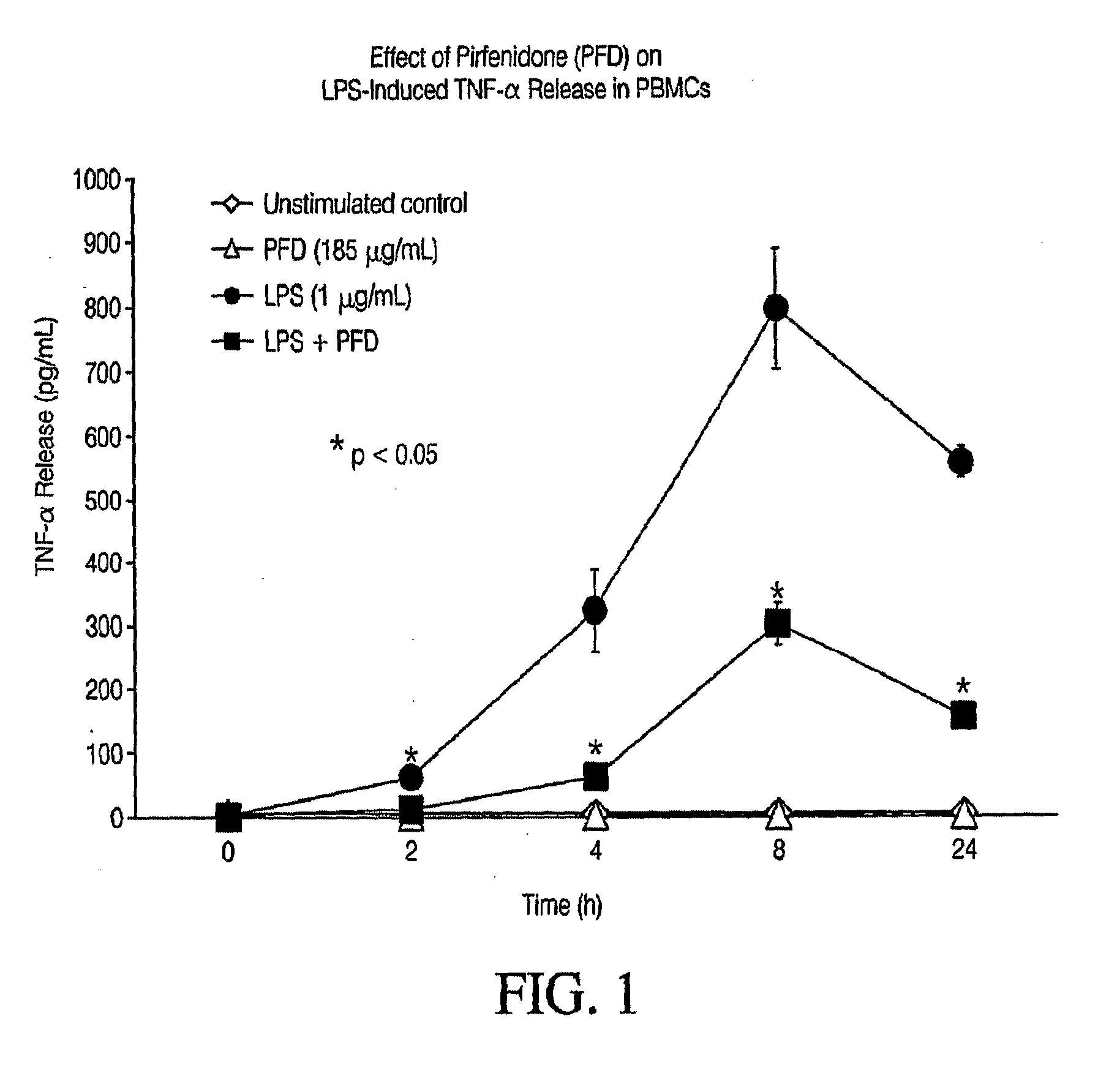
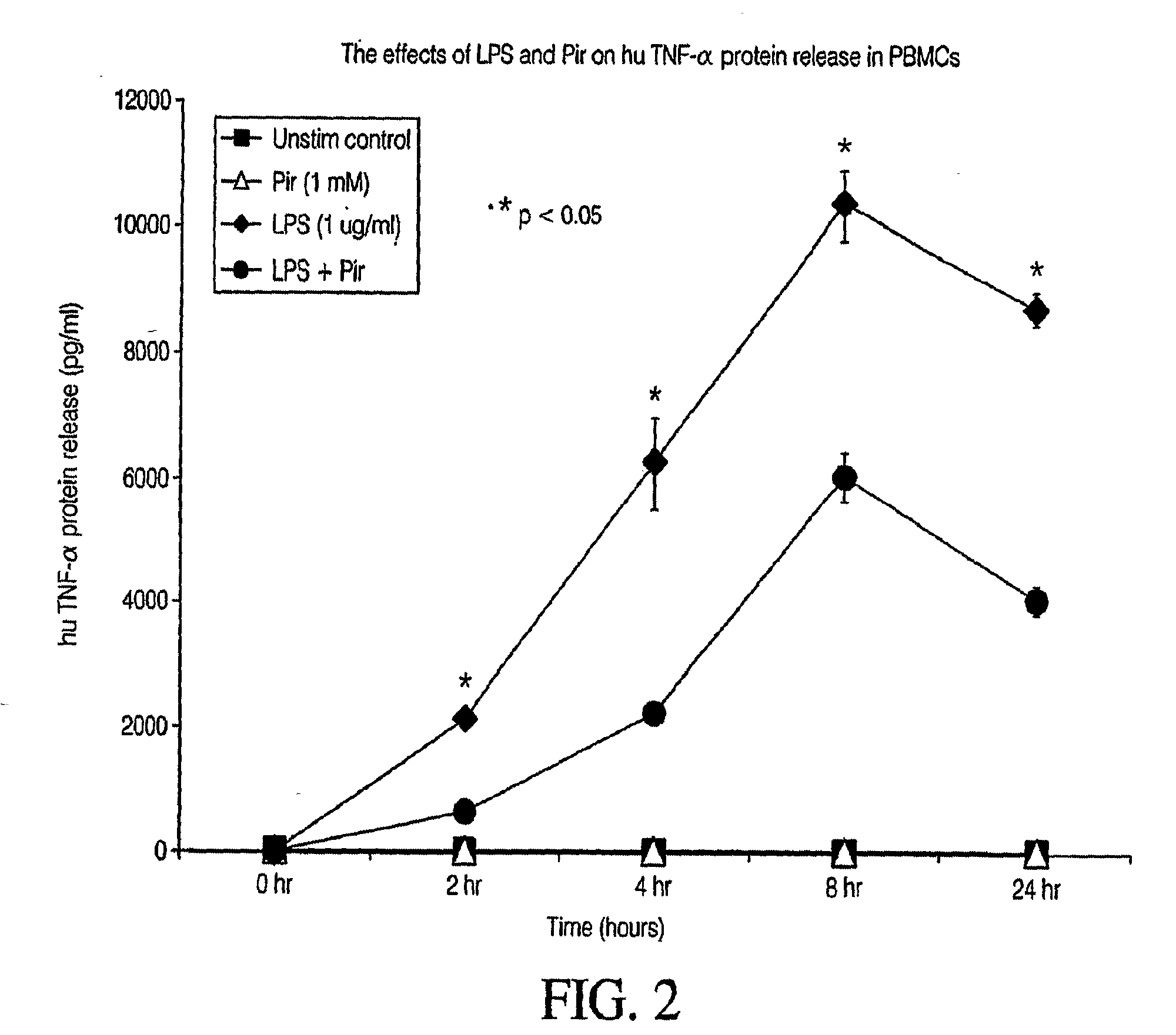
Pirfenidone/toll-like receptor (TLR) agonist compositions and methods for using them to stimulate production of granulocyte colonizing stimulating factor (g-csf)
InactiveUS20090170804A1Increase the number ofBiocideCarbohydrate active ingredientsPirfenidoneToll-like receptor
The invention disclosed herein relates to compositions and methods for treating subjects suffering from or at risk of developing neutropenia. In some embodiments, the methods comprise administering to a subject suffering from or at risk of developing neutropenia, an effective amount of pirfenidone and one or more toll-like receptor (TLR) agonists.
Owner:INTERMUNE INC
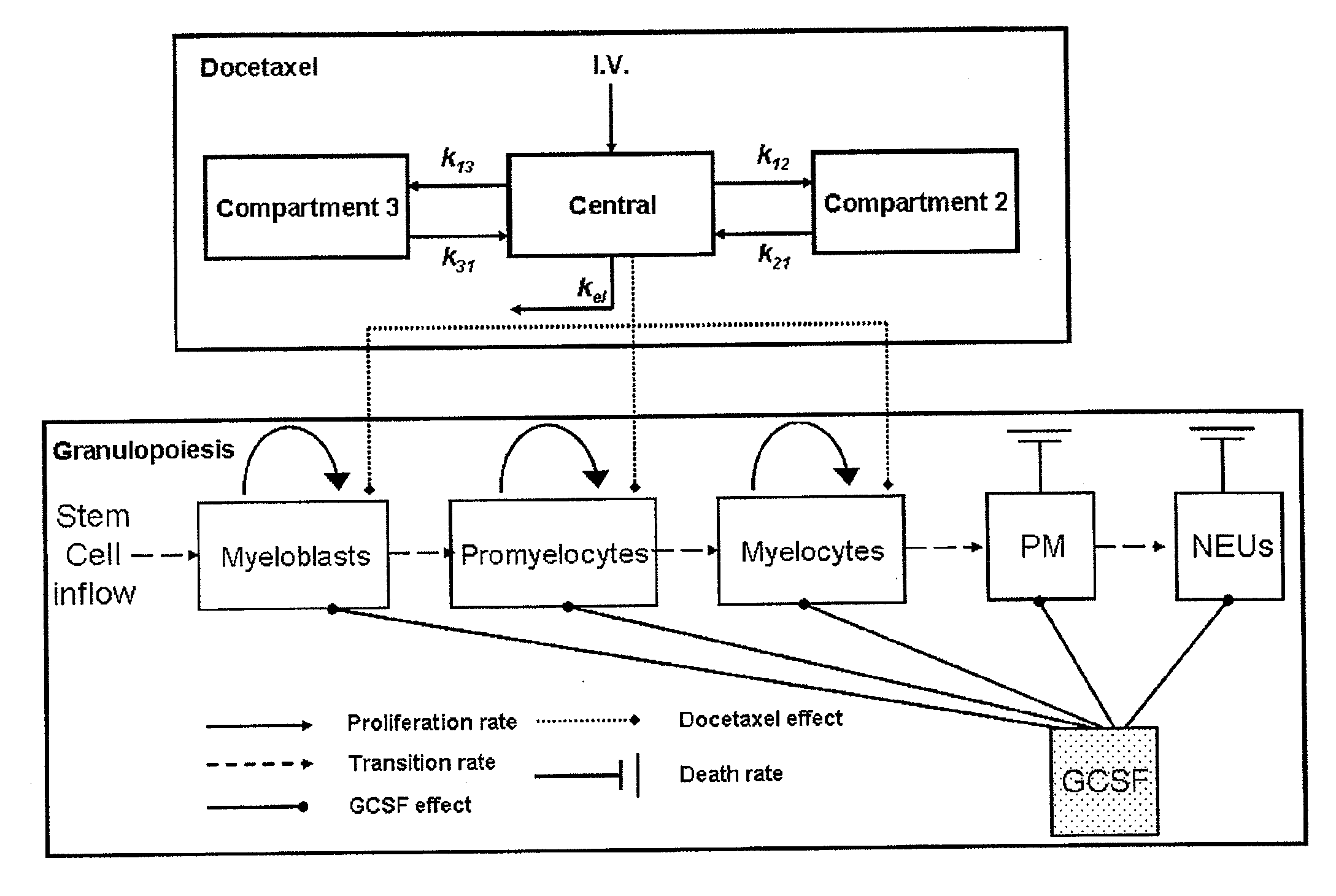
Cancer therapy by docetaxel and granulocyte colony-stimulating factor (g-csf)
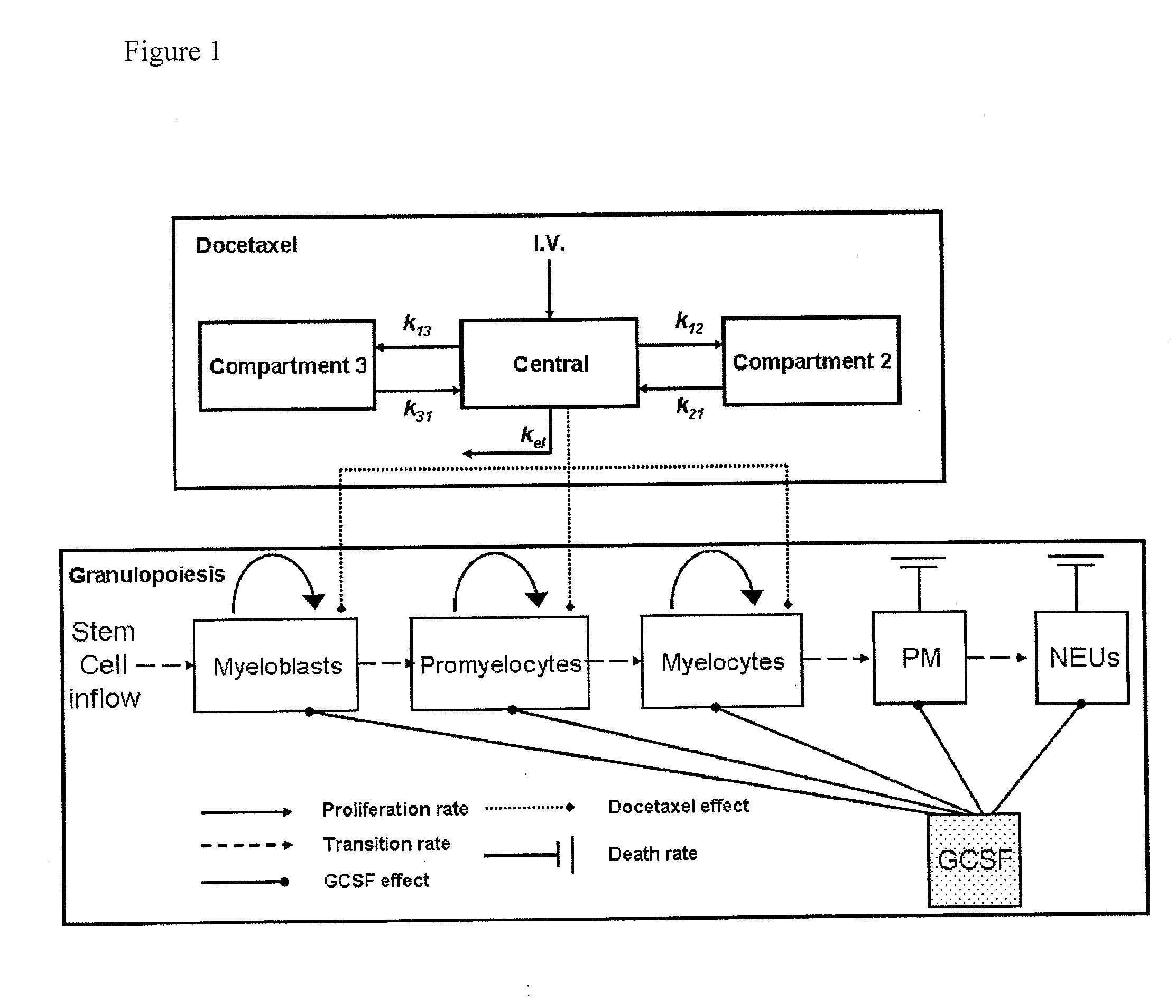
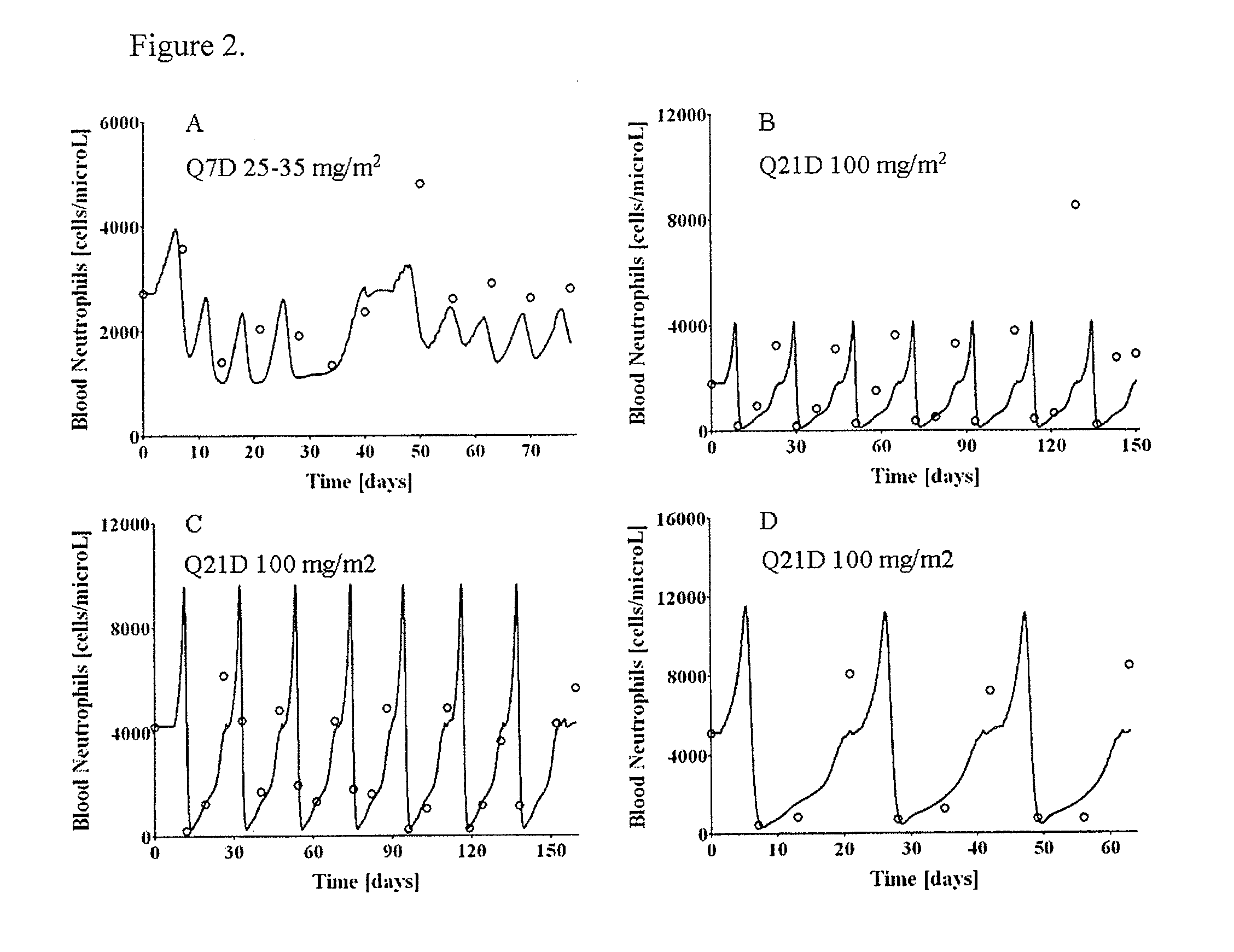
Neutropenia is the dose-limiting toxicity of the tri-weekly docetaxel (Taxotere®) schedule. Here, we evaluate in Metastatic Breast Cancer (MBC) patients (N=38) a computerized method for predicting docetaxel-induced neutropenia, and use the model to identify improved docetaxel and Granulocyte Colony Stimulating Factor (G-CSF) regimens. Pharmacokinetics / pharmacodynamics (PK / PD) models were created and simulated concomitantly with a mathematical granulopoiesis model. Individual baseline neutrophil counts and docetaxel schedules served as inputs. Our trial validated the model accuracy in predicting nadir timings (r=0.99), grade 3 / 4 neutropenia (86% success) and neutrophil profiles (r=0.62). Model was robust to CYP3A-induced variability, except for slightly less accurate grade 3 / 4 neutropenia predictions. Simulations confirm smaller toxicity of the weekly docetaxel regimen than the tri-weekly one, and suggest an optimal G-CSF support for alleviating neutropenia, 60 μg / day QD×3, 6-7 days post-docetaxel, administered tri- and bi-weekly, and 4 days post weekly docetaxel>33 mg / m2.
Owner:OPTIMATA
Sepsis Treatment Methods
Owner:HARBOR DIVERSIFIED +2
Pharmaceutical preparation containing recombinant human serum albumin-human granulocyte colony stimulating factor fusion protein and preparation thereof
ActiveCN102670522AStable in natureClinically convenientPowder deliveryPeptide/protein ingredientsFreeze-dryingSubcutaneous injection
The invention discloses a pharmaceutical preparation containing recombinant human serum albumin-human granulocyte colony stimulating factor fusion protein and preparation of the pharmaceutical preparation. According to the invention, the medical preparation containing the rHSA / G-CSF (recombinant human serum albumin-human granulocyte colony stimulating factor fusion protein) is a freeze-drying preparation, and each freeze-drying preparation comprises the following components: 1-30mg of rHSA / G-CSF protein, 10-80mg of pharmaceutically acceptable water-soluble excipient, 5-30mg of pharmaceutically acceptable protective agent and 5-50 mu mol of pH regulator. The pharmaceutical preparation containing the rHSA / G-CSF protein is suitable for administration in the ways of subcutaneous injection or intravenous injection and the like after being dissolved in water for injection, and the pharmaceutical preparation can be injected for the right dosage for treatment of neutrophilic granulocytopenia.
Owner:JIANGSU T MAB BIOPHARMA
Pharmaceutical compositions
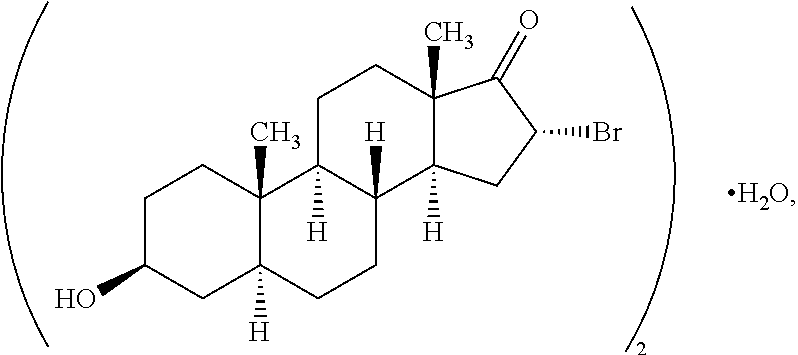
The invention relates to the use of compounds to ameliorate or treat an condition such as a cystic fibrosis, neutropenia or other exemplified conditions. Exemplary compounds that can be used include 3β-hydroxy-17β-aminoandrost-5-ene, 3β-hydroxy-16α-fluoro-17β-aminoandrost-5-ene, 3α-hydroxy-16α-fluoro-17β-aminoandrost-5-ene, 3β-hydroxy-16β-fluoro-17β-aminoandrost-5-ene, 1α,3β-dihydroxy-4α-fluoroandrost-5-ene-17-one, 1α,3β,17β-trihydroxy-4α-fluoroandrost-5-ene, 1β,3β-dihydroxy-6α-bromoandrost-5-ene, 1α-fluoro-3β,12α-dihydroxyandrost-5-ene-17-one, 1α-fluoro-3β,4α-dihydroxyandrost-5-ene and 4α-fluoro-3β,6α,17β-trihydroxyandrostane.
Owner:HOLLIS EDEN PHARMA +2
Method of Judging Risk for Onset of Drug-Induced Granulocytopenia
InactiveUS20070264631A1Sugar derivativesMicrobiological testing/measurementGranulocytopeniasInsulin receptor
Means for determining the presence of the risk of drug-induced granulocytopenia in a human is provided. A method for assessing the risk of drug-induced granulocytopenia, including detecting a polymorphism of the human insulin receptor substrate-2 gene of a subject, and determining the presence of the risk of drug-induced granulocytopenia of the subject by use of the genetic polymorphism as an index.
Owner:OTSUKA PHARM CO LTD
Method of treatment for feline leukemia virus infections
InactiveUS6350443B1Reduce feverReduce in quantityBiocidePeptide/protein ingredientsGranulocytopeniasInterferon alpha
A method of treatment for feline leukemia virus infections by continuously administering a feline interferon preparation containing a feline interferon as a main component daily to a cat is disclosed. As a feline interferon, a feline omega (omega)-interferon is preferably used, and more particularly, a recombinant interferon is preferably used. A method of treatment using a therapeutic agent containing a feline omega-interferon as a main component in accordance with the present invention is a novel and superior method suitable for treating feline leukemia virus infections, and in particular, for treating neutropenia.
Owner:TORAY IND INC
Method of reducing neutropenia
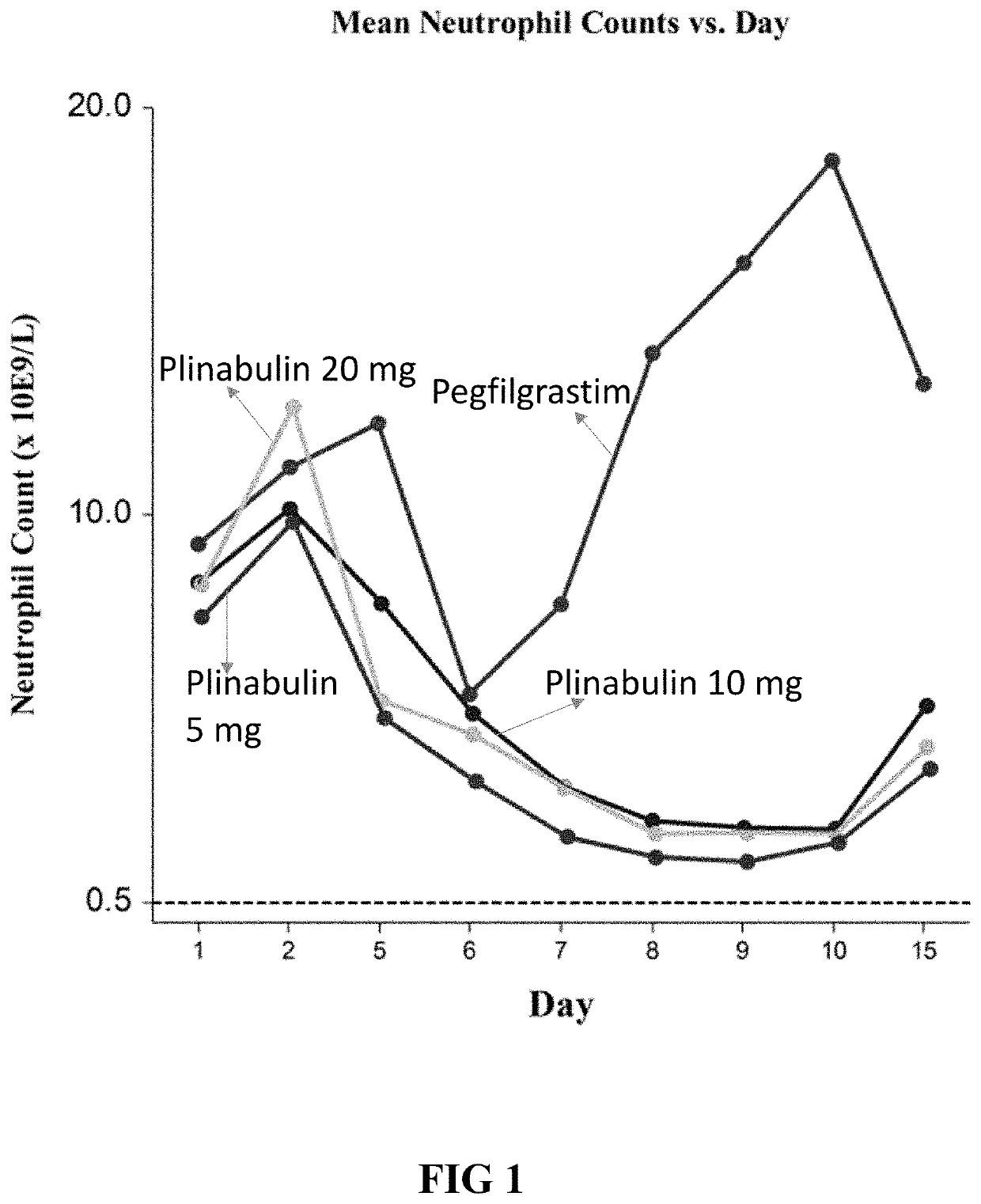
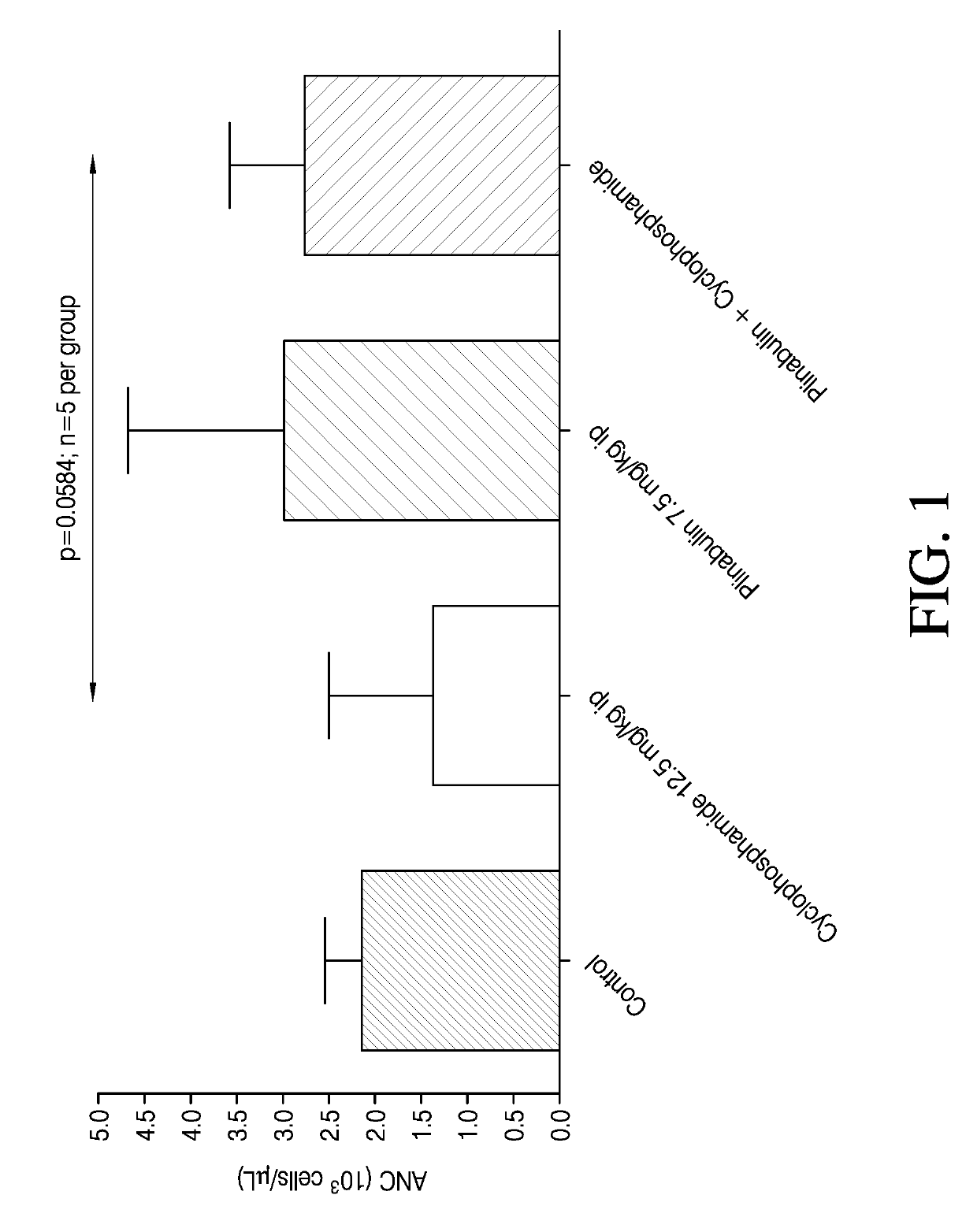
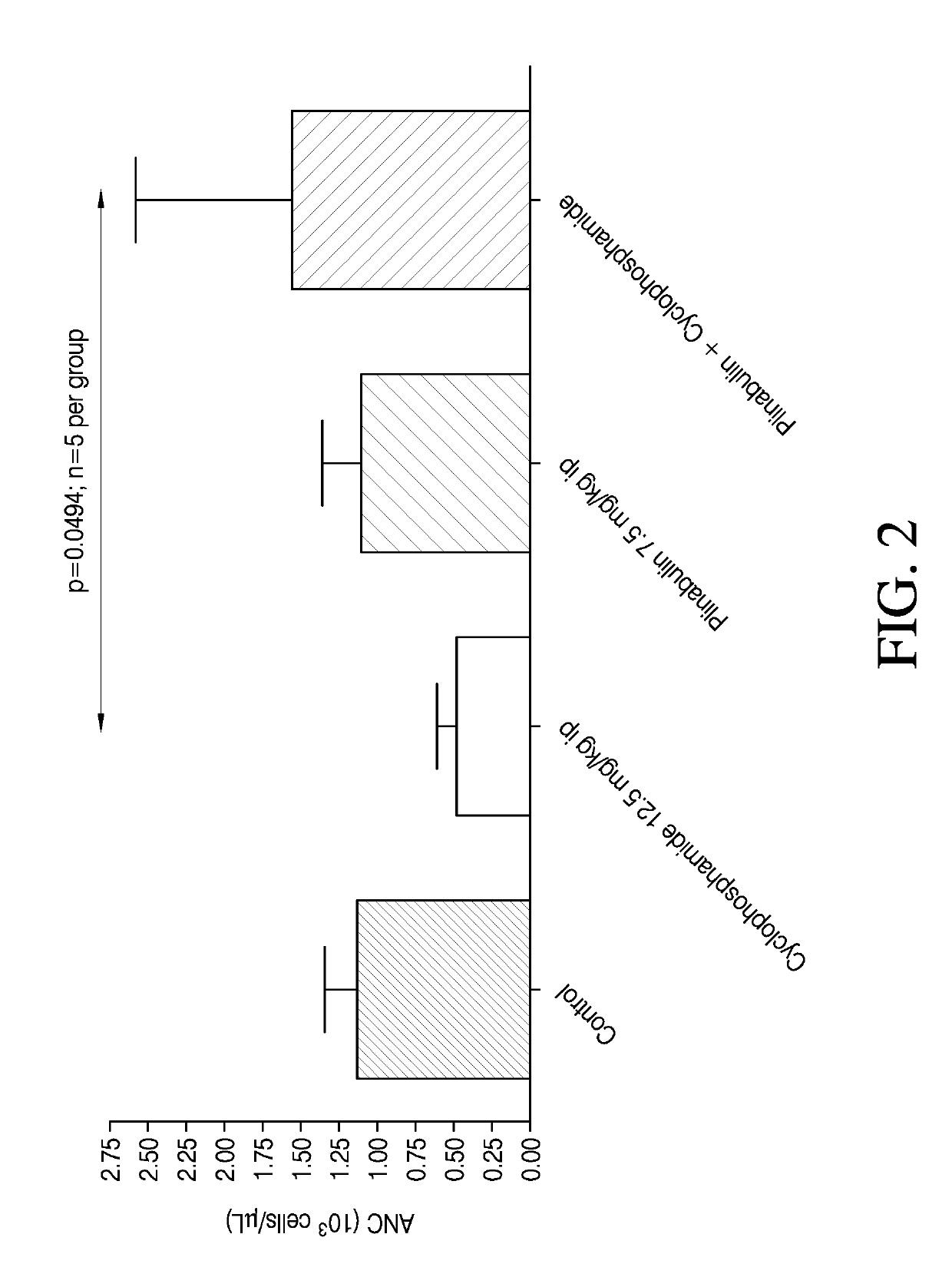
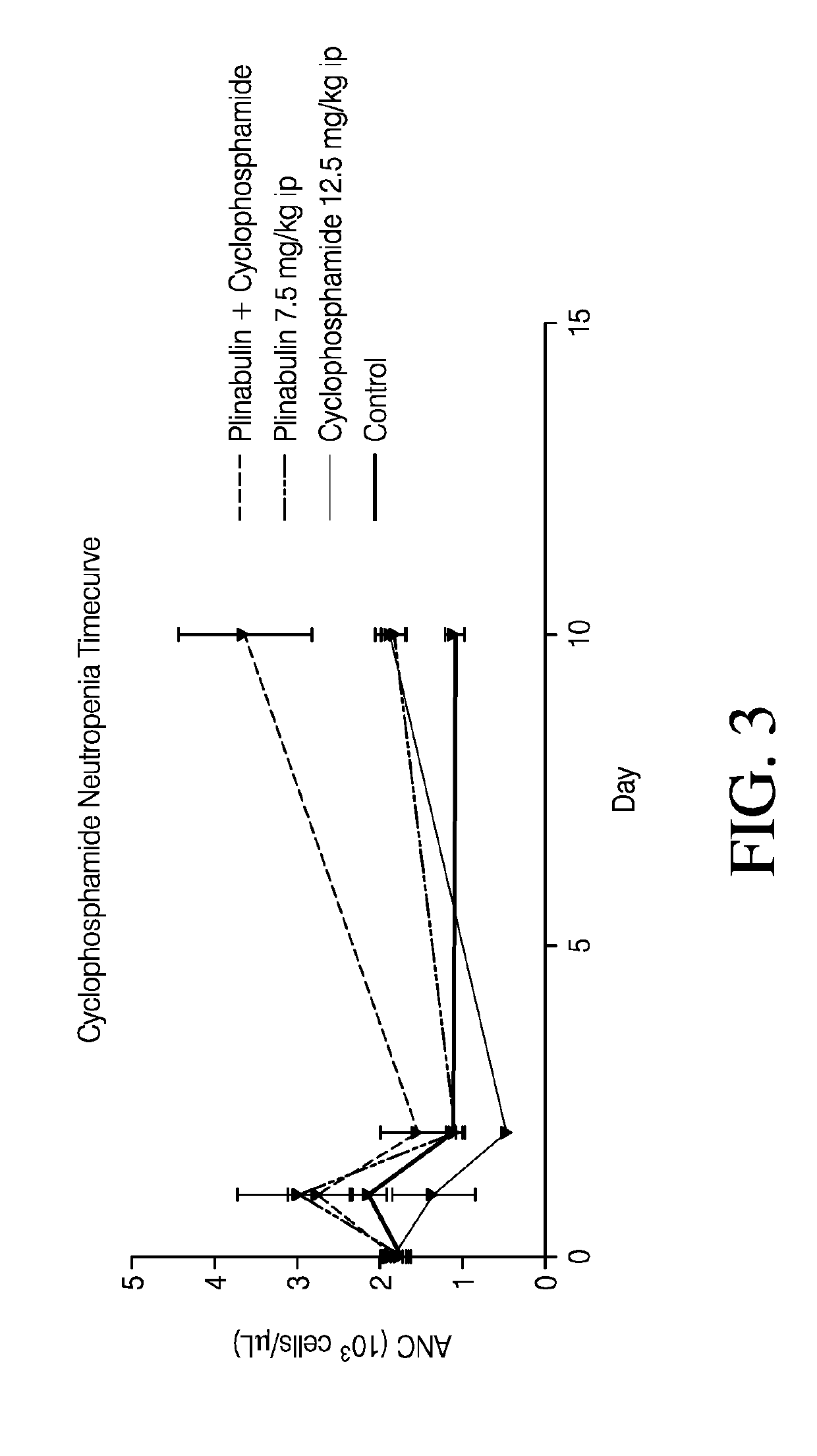
ActiveUS20200038395A1Alleviate and prevent neutrophil reductionOrganic active ingredientsPeptide/protein ingredientsPlinabulinGranulocytopenias
Disclosed herein are plinabulin and the use for reducing neutropenia. Some embodiments relate to reducing the docetaxel induced neutropenia using plinabulin.
Owner:BEYONDSPRING PHARMA INC
G-CSF (Granulocyte-Colony Stimulating Factor) fusion protein mutant, preparation method and application thereof
ActiveCN101824091AHigh activityExtended half-lifeFungiPeptide/protein ingredientsMulti siteHalf-life
The invention relates to a G-CSF (Granulocyte-Colony Stimulating Factor) fusion protein mutant, a preparation method and application thereof. The G-CSF fusion protein mutant is fusion protein having the effect of stimulating the proliferative activity of neutrophile granulocytes, and the structure of the G-CSF fusion protein mutant is G-CSF / carrier protein or carrier protein / G-CSF, wherein the G-CSF part contains multi-site mutations, which changes the activity and the receptor affinity. Compared with the traditional like products, the G-CSF fusion protein mutant has longer half-life period and higher biologic activity. Through injecting a proper dosage of the medicinal preparation, the neutrophilic granulocytopenia can be treated.
Owner:JIANGSU T MAB BIOPHARMA
Shp2 phosphatase inhibitors and methods of use thereof
ActiveUS20190307745A1Inhibiting SHP phosphatase activityOrganic chemistryAntineoplastic agentsDiseaseHER2 Positive Breast Cancer
The present invention relates to novel compounds and pharmaceutical compositions thereof, and methods for inhibiting the activity of SHP2 phosphatase with the compounds and compositions of the invention. The present invention further relates to, but is not limited to, methods for suppressing tumor cell growth, ameliorating the pathogenesis of systemic lupus erythematosus, and the treatment of various other disorders, including Noonan syndrome, diabetes, neutropenia, neuroblastoma, melanoma, juvenile leukemia, juvenile myelomonocytic leukemia, chronic myelomonocytic leukemia, acute myeloid leukemia, and other cancers associated with SHP2 deregulation with the compounds and compositions of the invention, alone or in combination with other treatments. Other cancers associated with SHP2 deregulation include HER2-positive breast cancer, triple-negative breast cancer, ductal carcinoma of the breast, invasive ductal carcinoma of the breast, non-small cell lung cancer, esophageal cancer, gastric cancer, squamous-cell carcinoma of the head and neck (SCCHN), and colon cancer.
Owner:D E SHAW RES & DEV LLC +1
Adjuvant immunotherapy for the treatment cancer, of clinical manifestations associated with the diseases like cachexia and correction of adverse effects of drugs such as immunosuppression, secundary cachexia, neutropenia and lymphopenia, comprising the association or combination of a biological response modifier specially selected and other substances with antineoplastic action and/or other treatments
InactiveUS20160166683A1Maximize the effect of treatmentMinimize of functionalityHeavy metal active ingredientsRadioactive preparation carriersAnticarcinogenGranulocytopenias
A compound for use in a method of treatment of cancer, including precancerous lesions, and adverse events caused by the disease or anti-cancer agents and treatments, such as cancer cachexia, lymphopenia, neutropenia, febrile neutropenia includes in combination an immunomodulatory and at least one anti-cancer agent or treatment suitable for treating the disease. The immunomodulator is a proteic aggregate of ammonium and magnesium phospholinoleate-palmitoleate anhydride. The anti-cancer agent or treatment suitable for treating the disease provides synergistic effects without additional toxicity when used with the immunomodulatory. The anti-cancer treatment is selected from the following group: surgical procedures, transplantation of bone marrow cells, systemic and localized radiotherapy, and combinations thereof.
Owner:NUNES LSEU SILVA
Compositions and treatment methods-4
The invention relates to the use of compounds to ameliorate or treat an condition such as a cystic fibrosis, neutropenia or other exemplified conditions. Exemplary compounds that can be used include 3β-hydroxy-17β-aminoandrost-5-ene, 3β-hydroxy-16α-fluoro-17β-aminoandrost-5-ene, 3α-hydroxy-16α-fluoro-17β-aminoandrost-5-ene, 3β-hydroxy-16β-fluoro-17β-aminoandrost-5-ene, 1α,3β-dihydroxy-4α-fluoroandrost-5-ene-17-one, 1α,3β,17β-trihydroxy-4α-fluoroandrost-5-ene, 1β,3β-dihydroxy-6α-bromoandrost-5-ene, 1α-fluoro-3β,12α-dihydroxyandrost-5-ene-17-one, 1α-fluoro-3β,4α-dihydroxyandrost-5-ene and 4α-fluoro-3β,6α,17β-trihydroxyandrostane.
Owner:BIOVIE INC
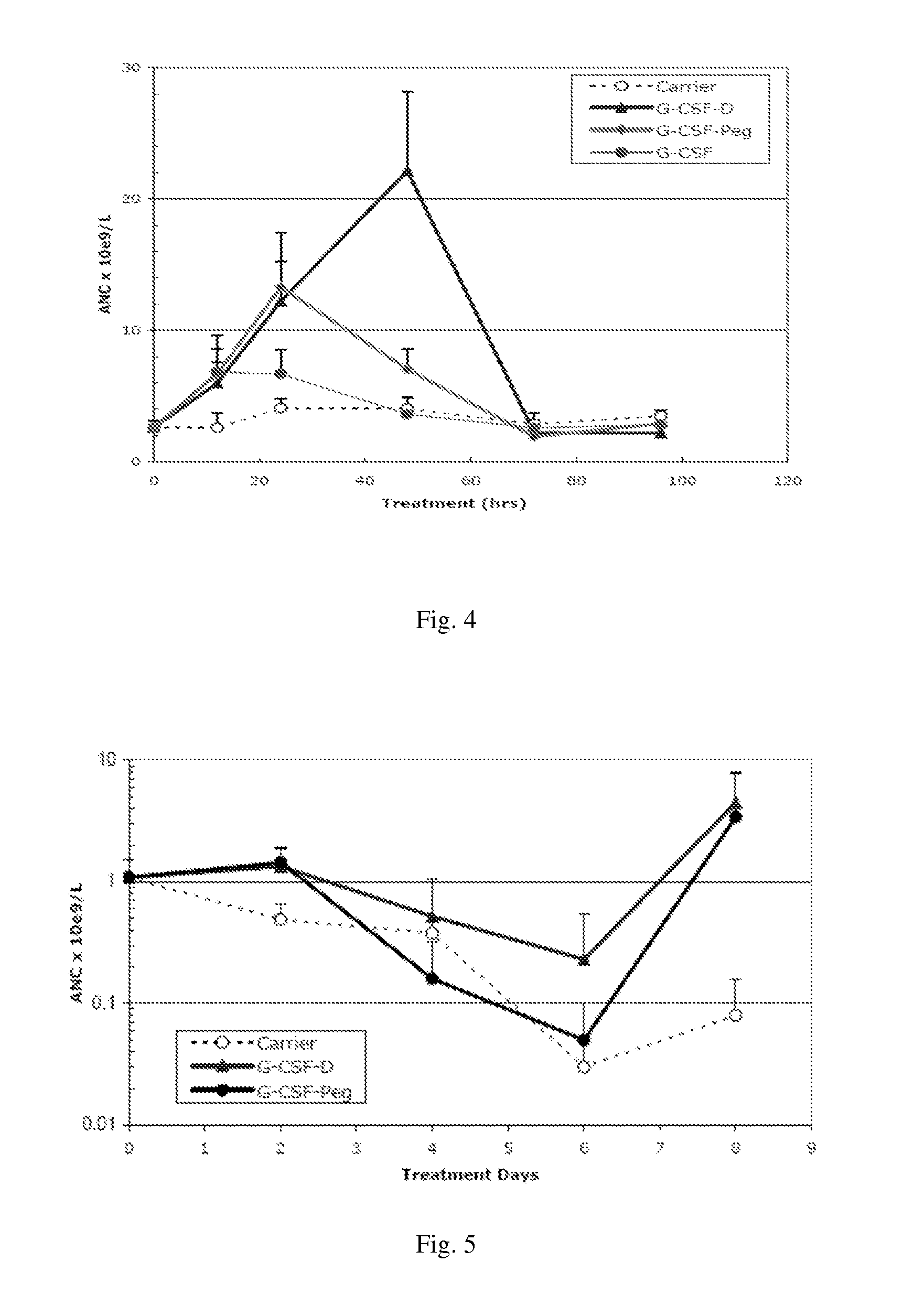
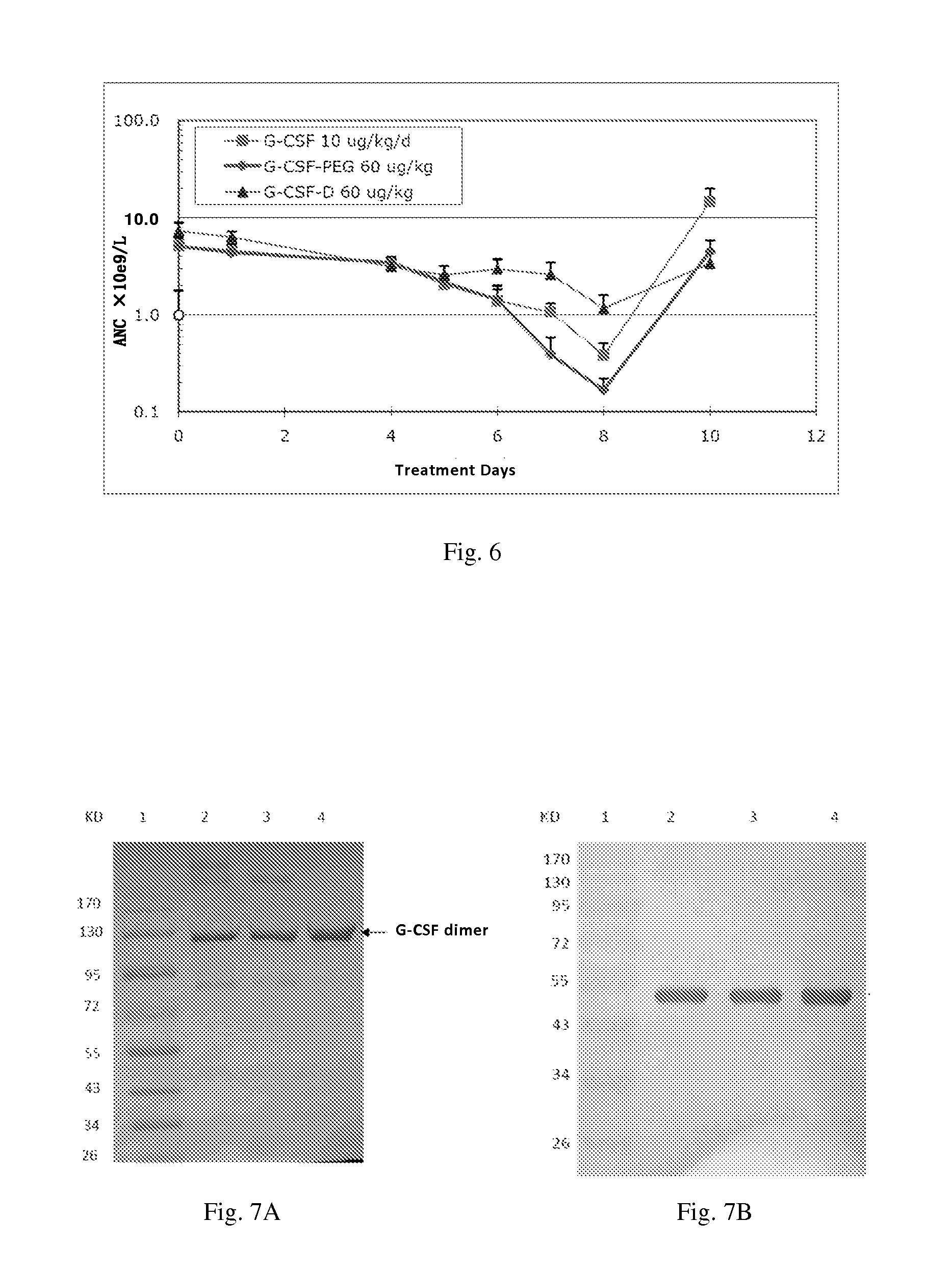
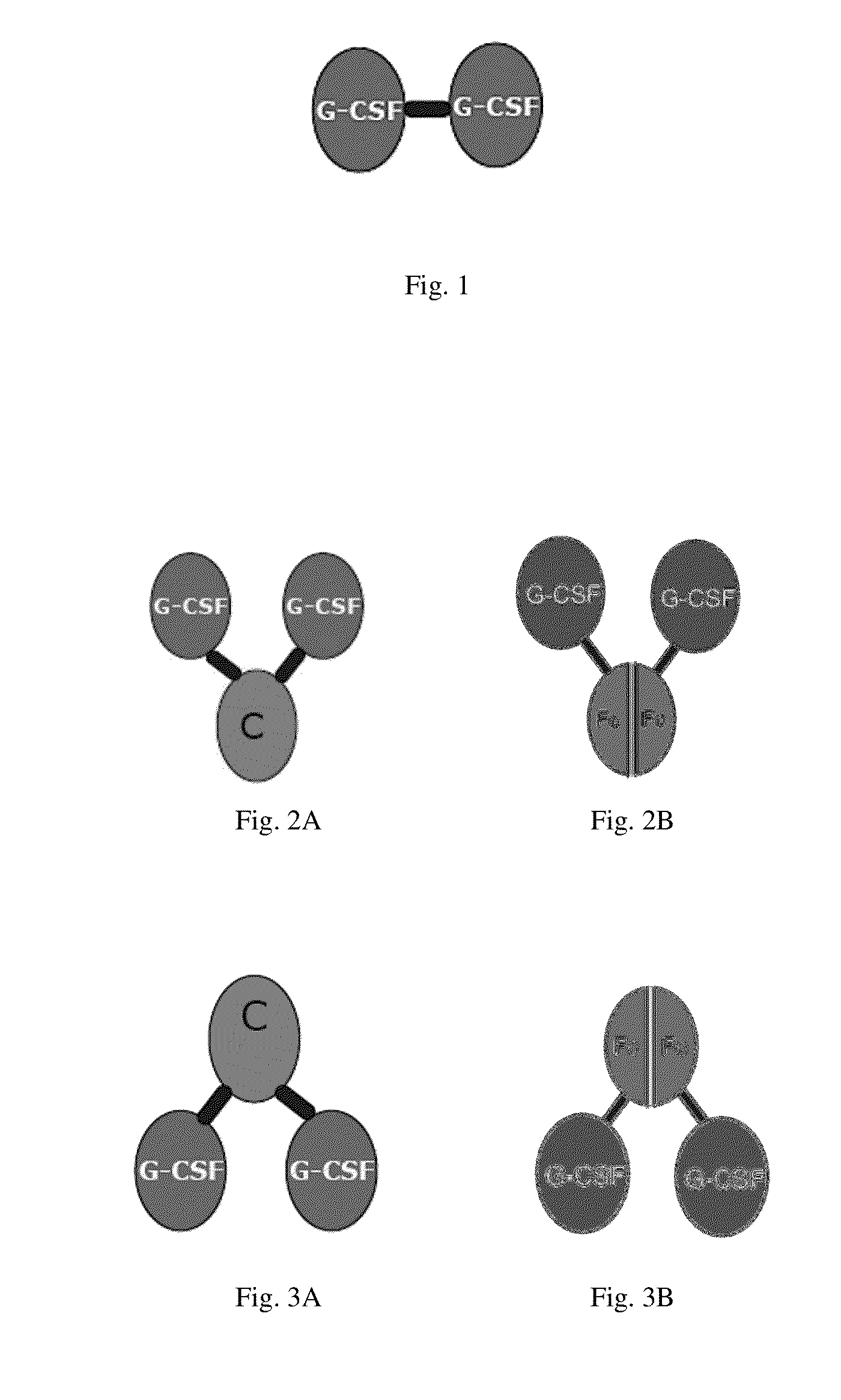
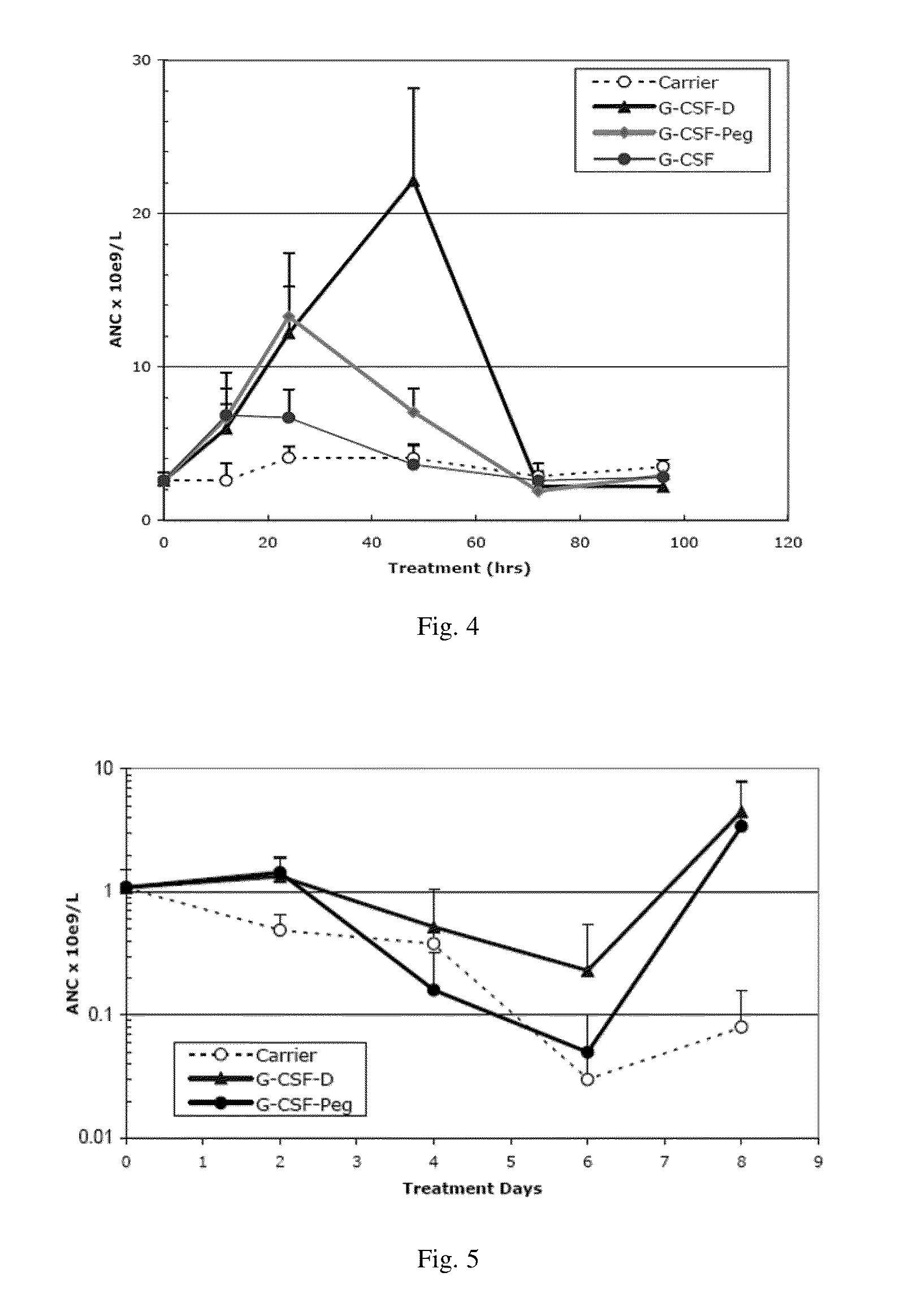
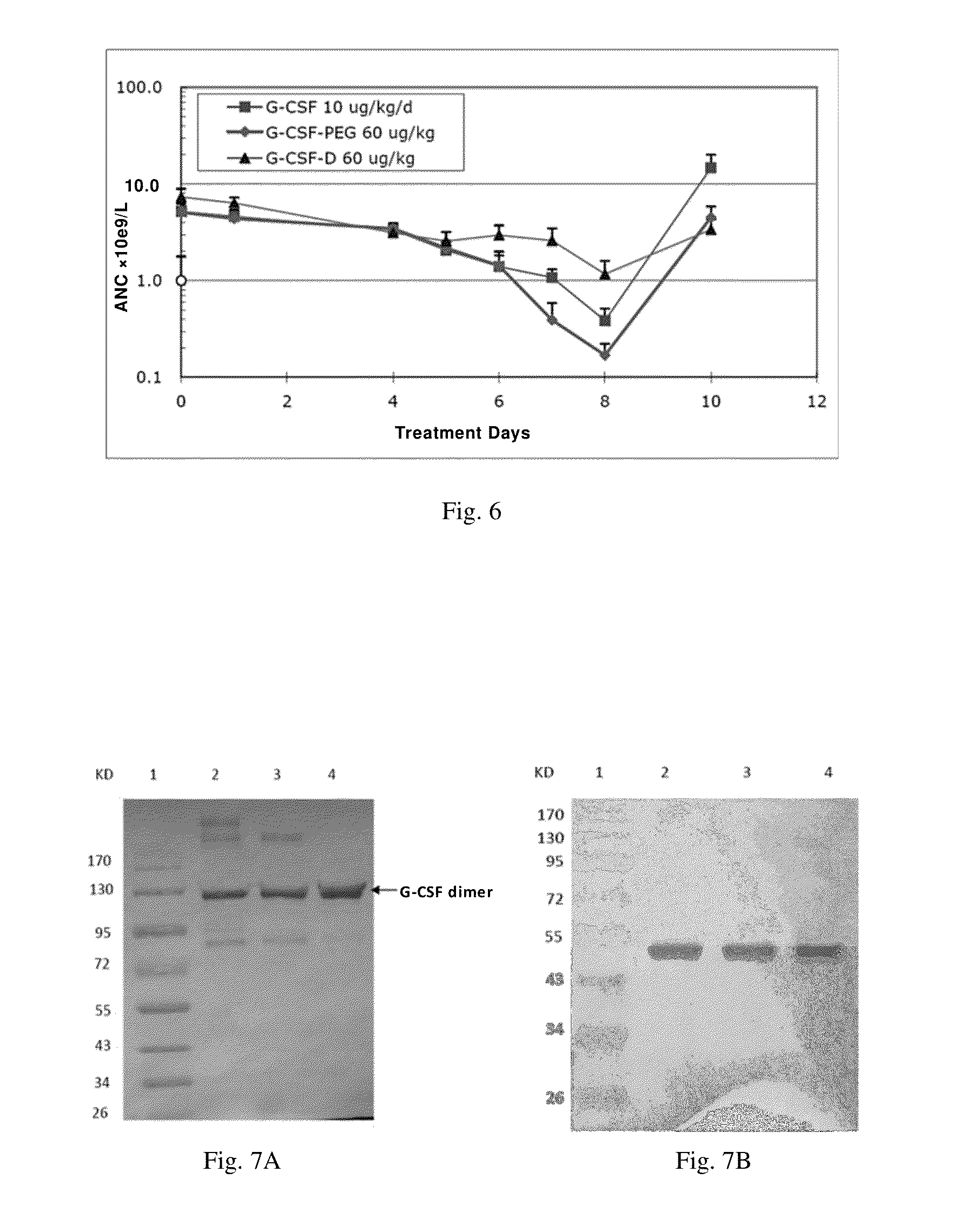
Use of g-csf dimer in the treatment of neutropenia
InactiveUS20130165637A1Good curative effectHybrid immunoglobulinsPeptide/protein ingredientsMedicineHalf-life
This invention relates to a use of G-CSF dimer in the treatment of neutropenia. In particular, the recombinant human G-CSF of the present invention can enhance the differentiation and development of neutrophils in animal, and thus effectively reduce the severity of the severe neutropenia and shorten the time of severe neutropenia for the post-chemotherapy cancer patients. Serum half-life of G-CSF dimer of this invention is prolonged and the biological activity thereof is increased, providing a better effect in the treatment of neutropenia.
Owner:EVIVE BIOTECHNOLOGY (SHANGHAI) LTD
Composition and method for reducing neutropenia
ActiveUS20190175587A1Reducing neutropeniaHeavy metal active ingredientsOrganic active ingredientsPlinabulinGranulocytopenias
Disclosed herein are methods of reducing neutropenia that is caused by chemotherapy or radiation therapy by administering plinabulin to a subject.
Owner:BEYONDSPRING PHARMA INC
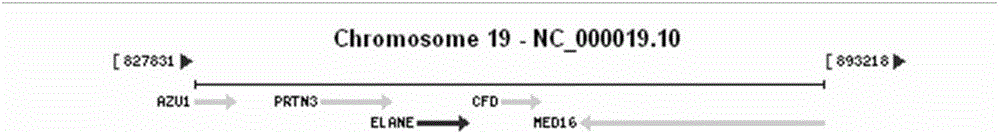
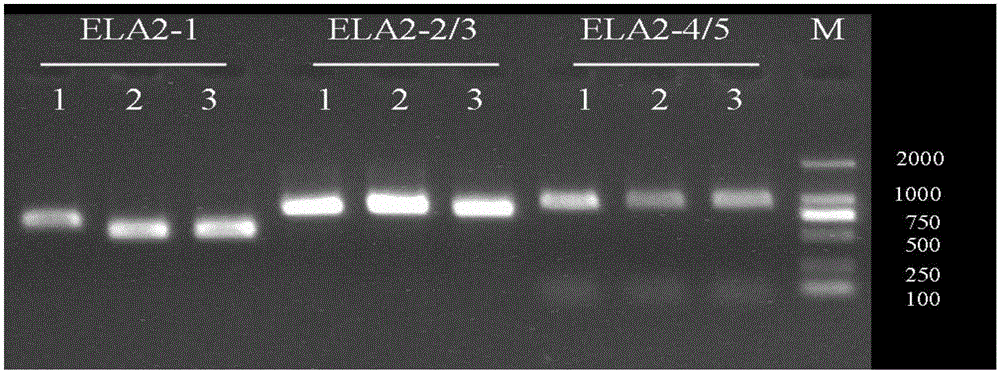
Method and primer for detecting ELA2 gene
InactiveCN106222287AGood amplification efficiencyGuaranteed accuracyMicrobiological testing/measurementDNA/RNA fragmentationPrenatal diagnosisGranulocytopenias
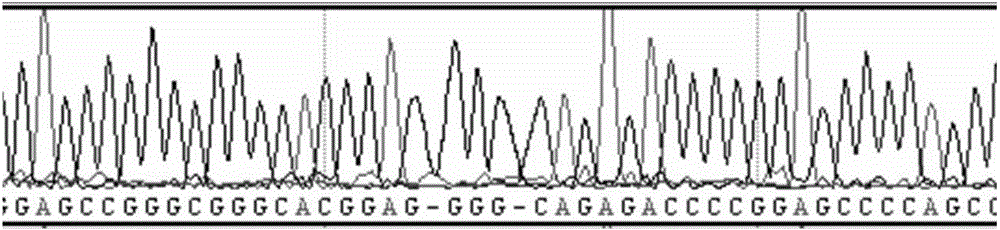
The invention discloses a primer and a method for detecting mutation of an ELA2 gene of a patient with congenital neutropenia. The primer comprises a primer which is used for amplifying the whole exon sequence of the ELA2 gene; the Sanger sequencing technology and a sequencing primer are adopted. According to the method and the primer, the mutation of the whole exon sequence of the ELA2 gene in the body of the patient with the congenital neutropenia can be detected rapidly. The detection result finished by the method and the primer is accurate, can assist in diagnosing the congenital neutropenia, and has an important reference meaning for early intervention, early treatment and antenatal diagnosis.
Owner:WUHAN ADICON CLINICAL LAB
Compounds for the treatment of inflammation and neutropenia
InactiveUS20130059773A1Affect survivalEliminate the effects ofAntibacterial agentsCompound screeningDiseaseMedicine
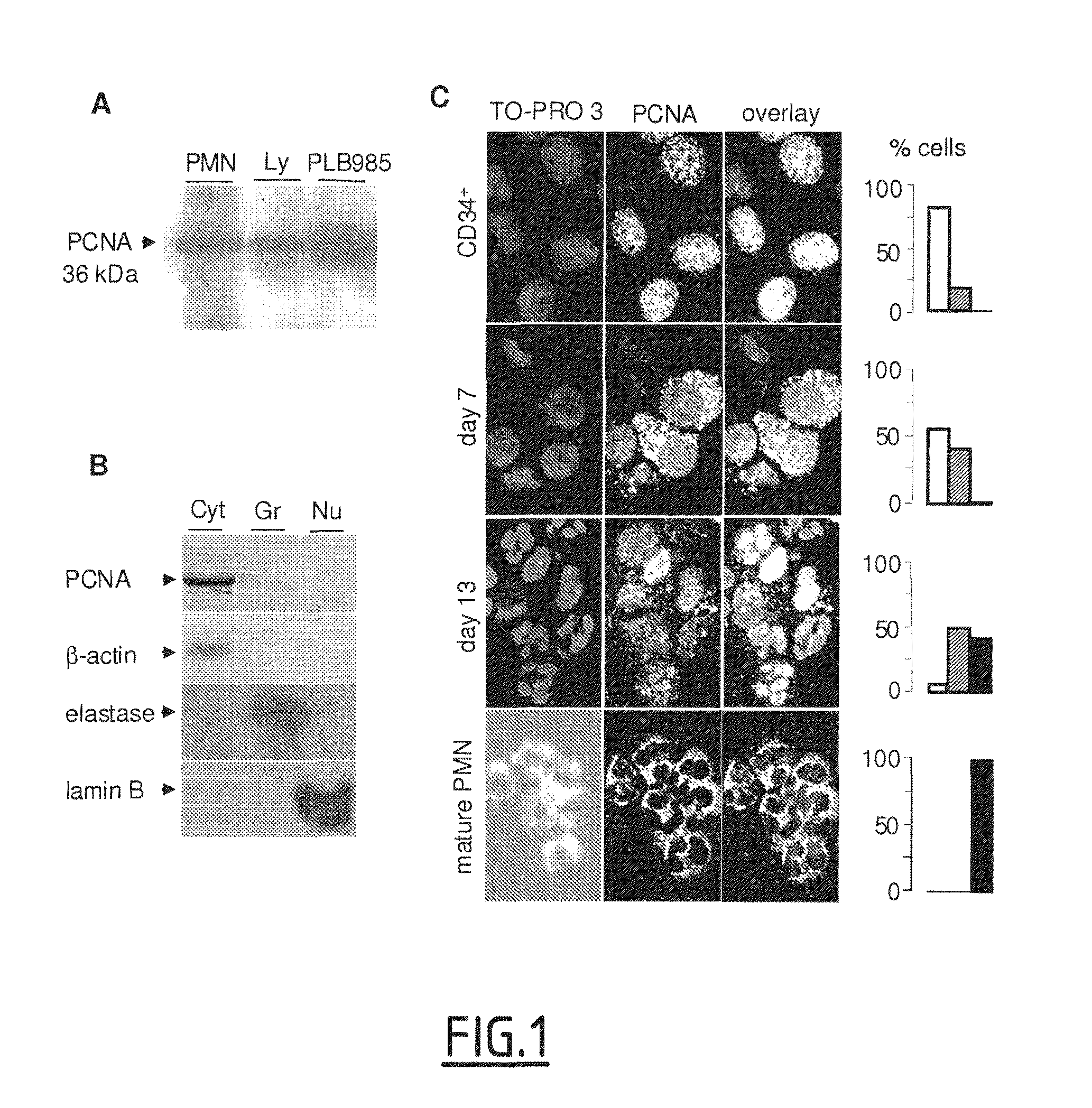
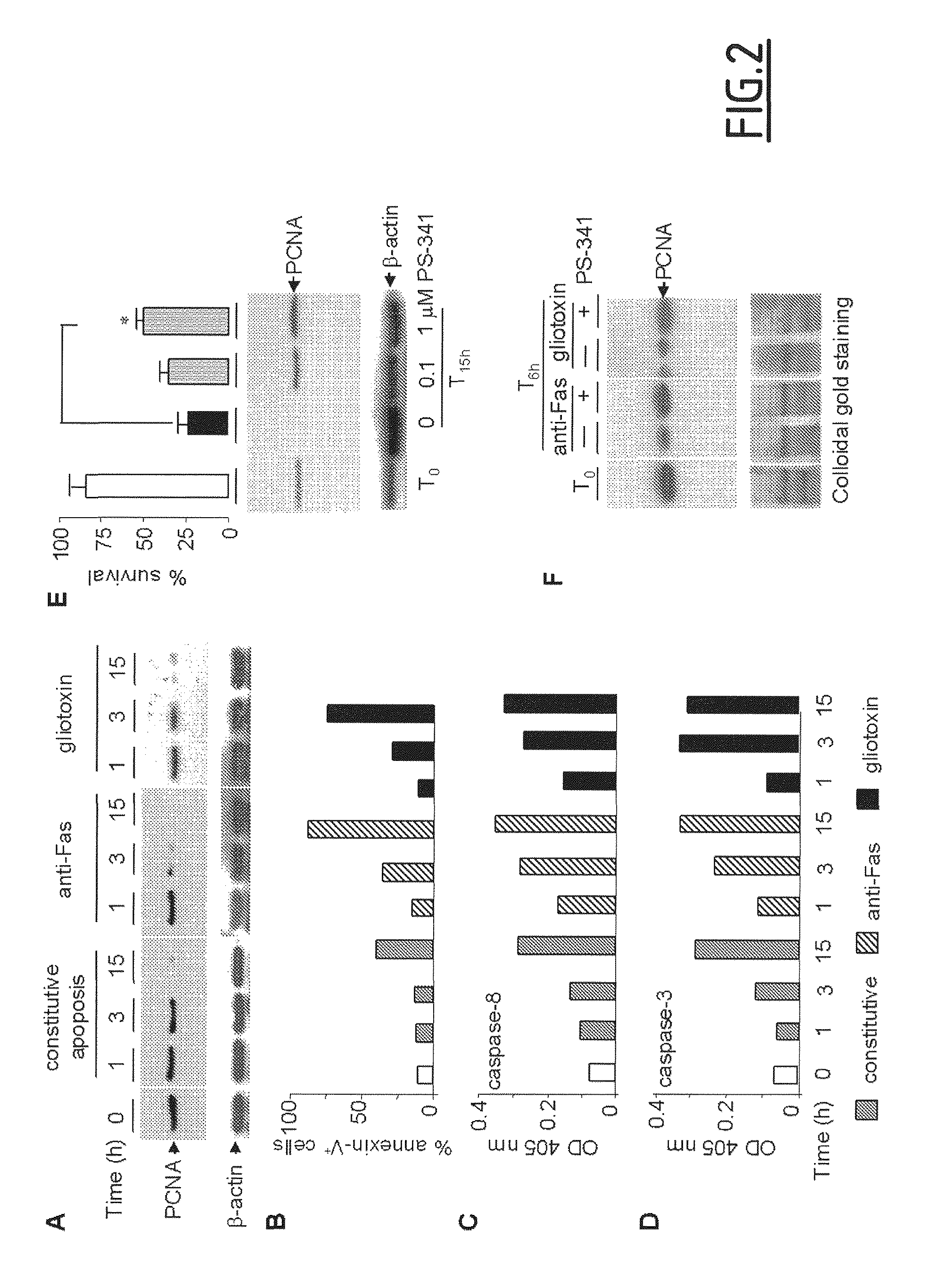
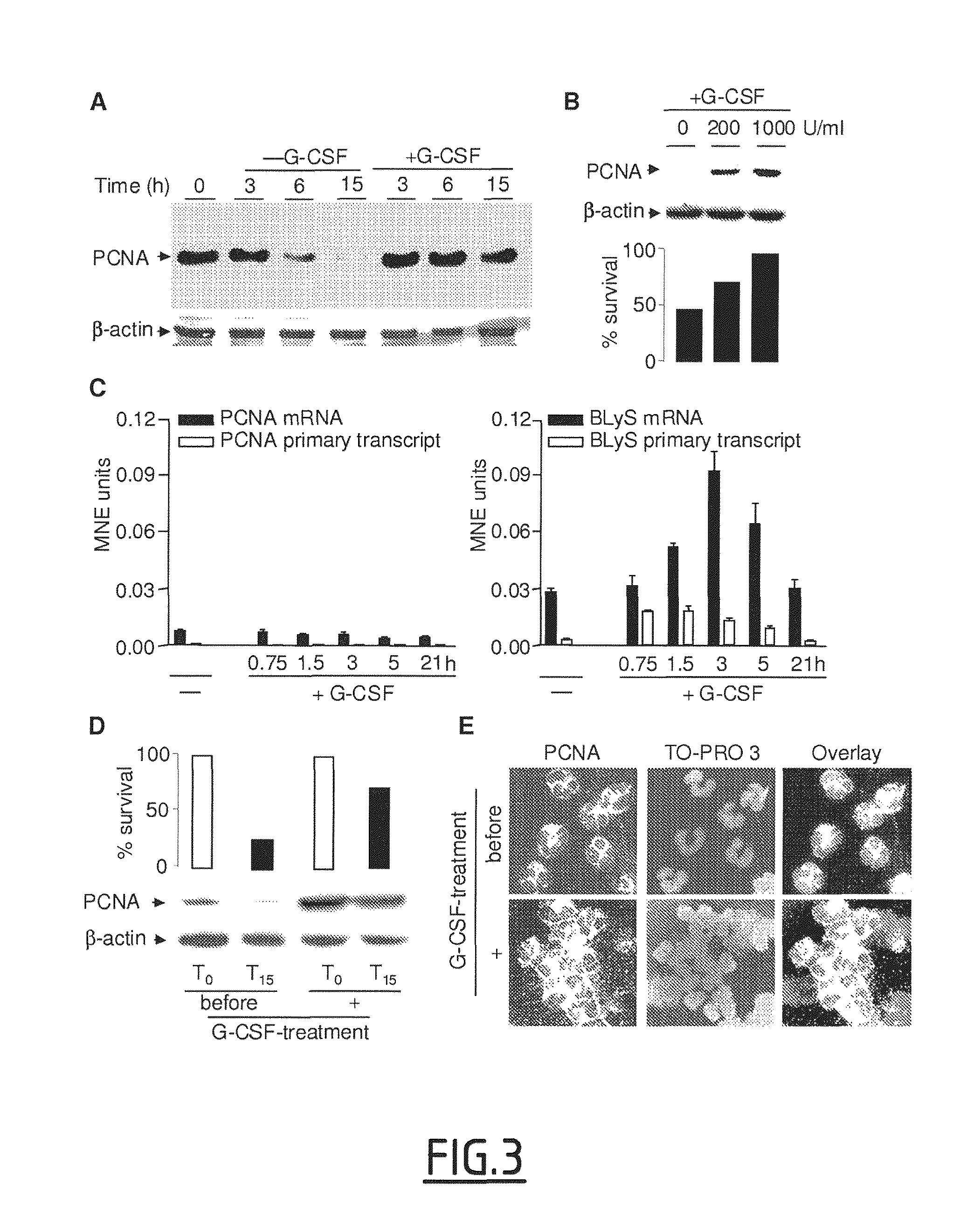
The present invention concerns compounds modulating apoptosis of neutrophil cells. In particular, the invention concerns compounds inhibiting an interaction between Proliferating Cell Nuclear Antigen (PCNA) and proteins binding to cytoplasmic PCNA in neutrophil cells, for use in the treatment of a disease involving a neutrophil-dependent inflammatory process. The invention also relates to a method for the identification of a compound for use in the treatment of a neutrophil-dependent inflammatory process. The invention further relates to peptides for use in the treatment of neutropenia.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Nitrogen-terminal fixed-point coupling method for colony stimulating factor of column chromatography granulocyte and coupled product
ActiveCN101585864APeptide/protein ingredientsPeptide preparation methodsReaction rateColony-stimulating factor
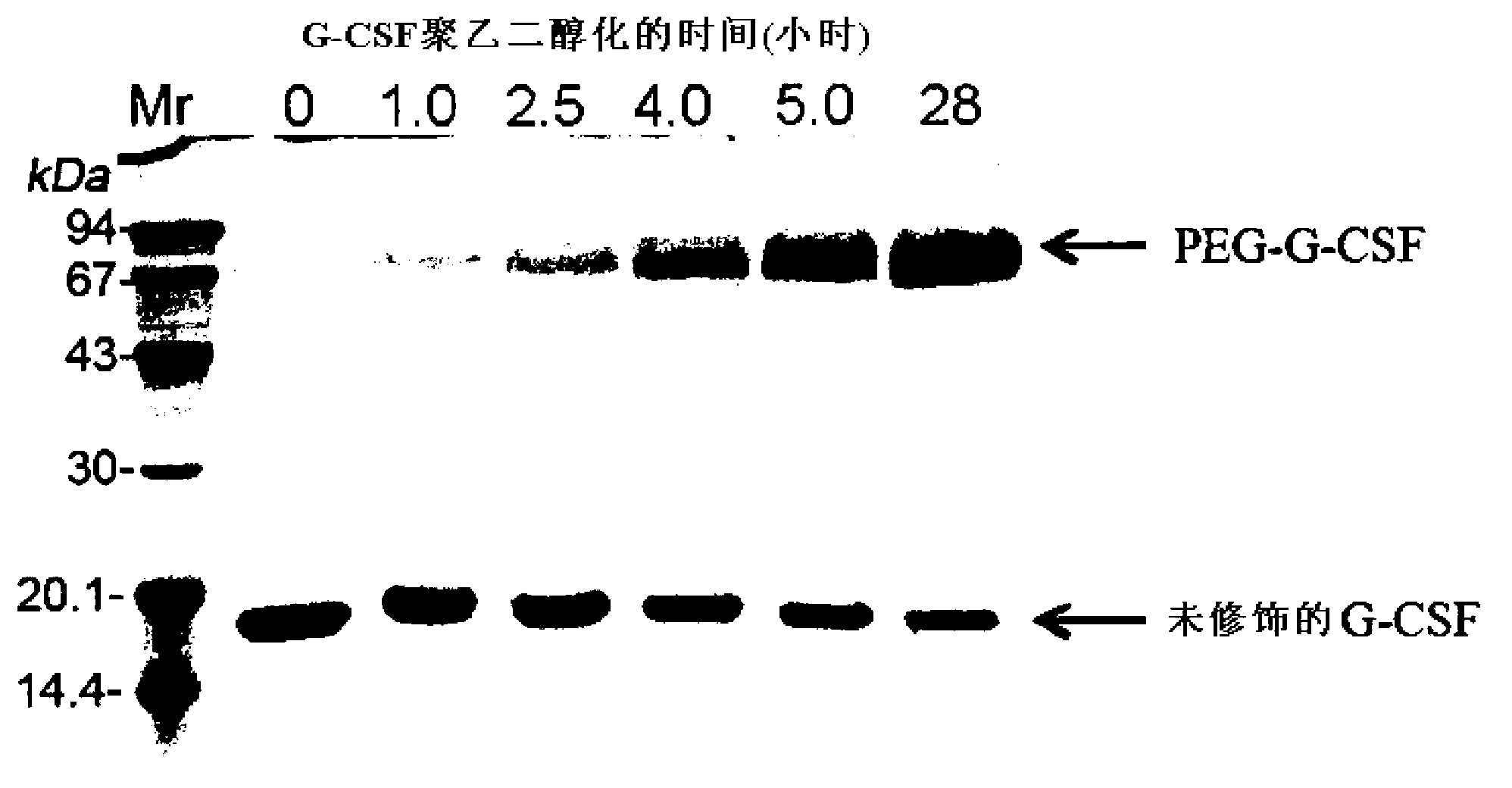
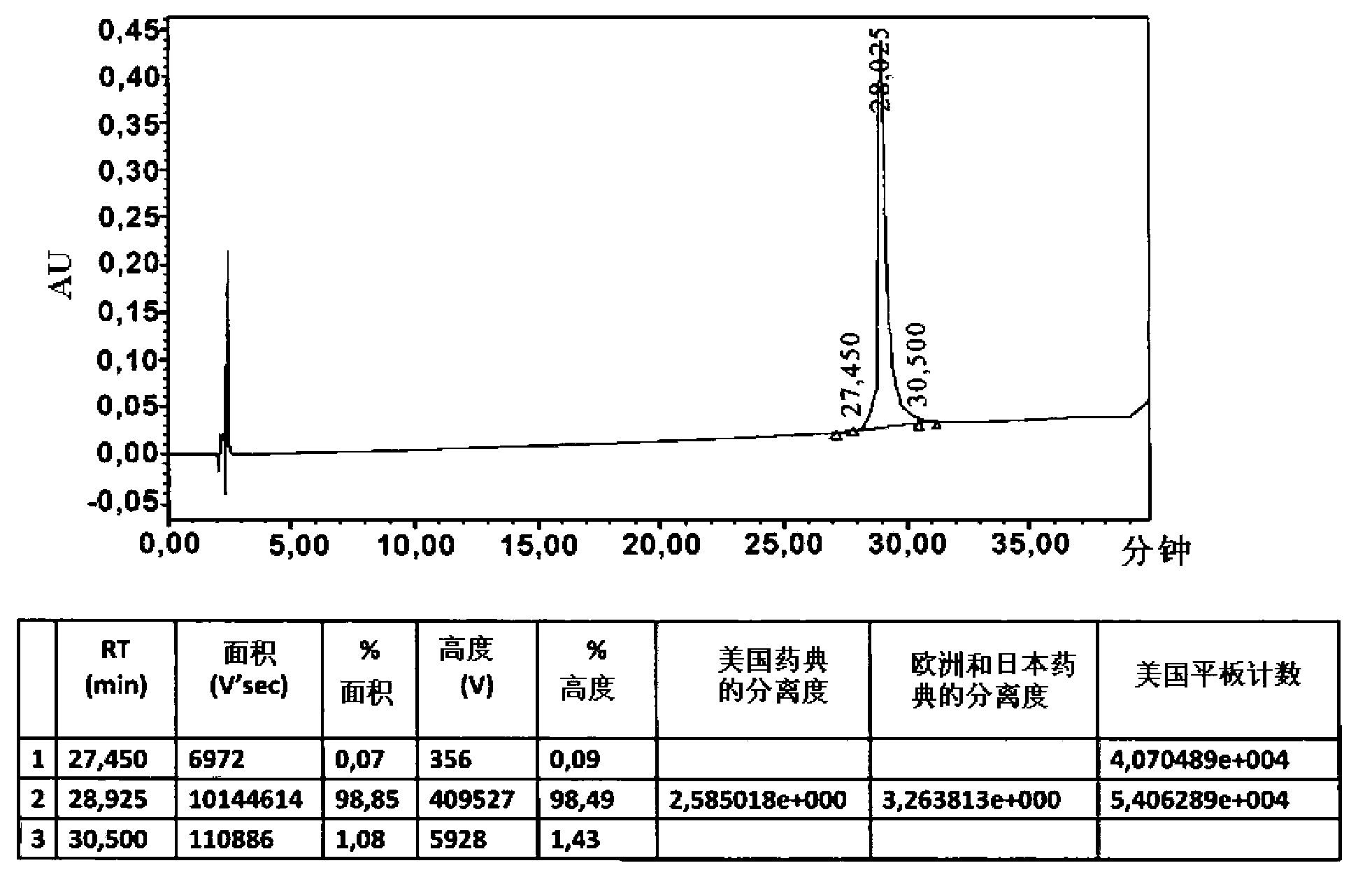
The invention discloses a method for the fixed-point coupling of polyethylene glycol on a nitrogen-terminal of a colony stimulating factor of a column chromatography granulocyte, wherein the coupling method comprises the following steps that: 1) a chromatography medium is preferred to be an exchange medium; 2) the height of a column bed of a chromatography column is preferred to be the upper limit of the recommended height of the column; 3) the sample volume of rhG-CSF protein is preferred to be 30 to 50 percent of the actually measured maximum loading capacity of the medium; 4) the activated mPEG is sampled under conditions of lower sample flow rate, longer sample time (3 to 5 hours) and higher pH value of reaction rate; 5) the sample volume of the activated mPEG is made according to the reaction volume ratio of the activated mPEG to the rhG-CSF of 1-50mol:1mol; and 6) 0.5 to 1M of salt solution of sodium chloride is used for gradient elution. The invention also discloses a coupling compound prepared by the method, which is a pegylation recombination human granulocyte colony stimulating factor, of which the structural formula is CH3-(CH2CH2-O)n-(CH2)r-NH-rhG-CSF or CH3-(CH2-CH2-O)n-(CH2)r-NH-rhG-CSFm, wherein n is between 570 and 2,200, and r is between 1 and 3; moreover, the invention also discloses application of the coupling compound in the preparation of a preparation preventing and treating the granulocytopenia.
Owner:TIANJIN PAIGE BIOTECHNOLOGY CO LTD +1
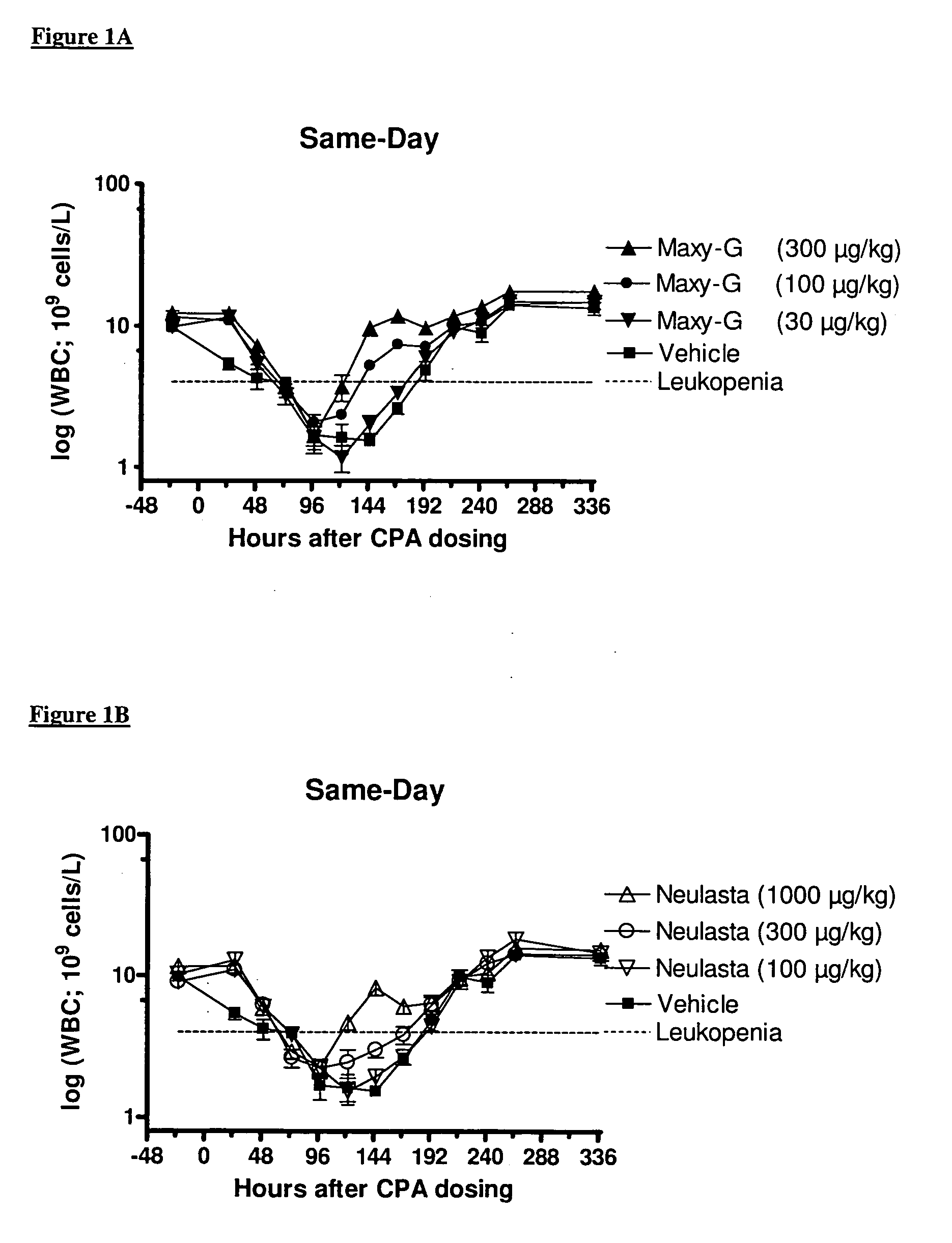
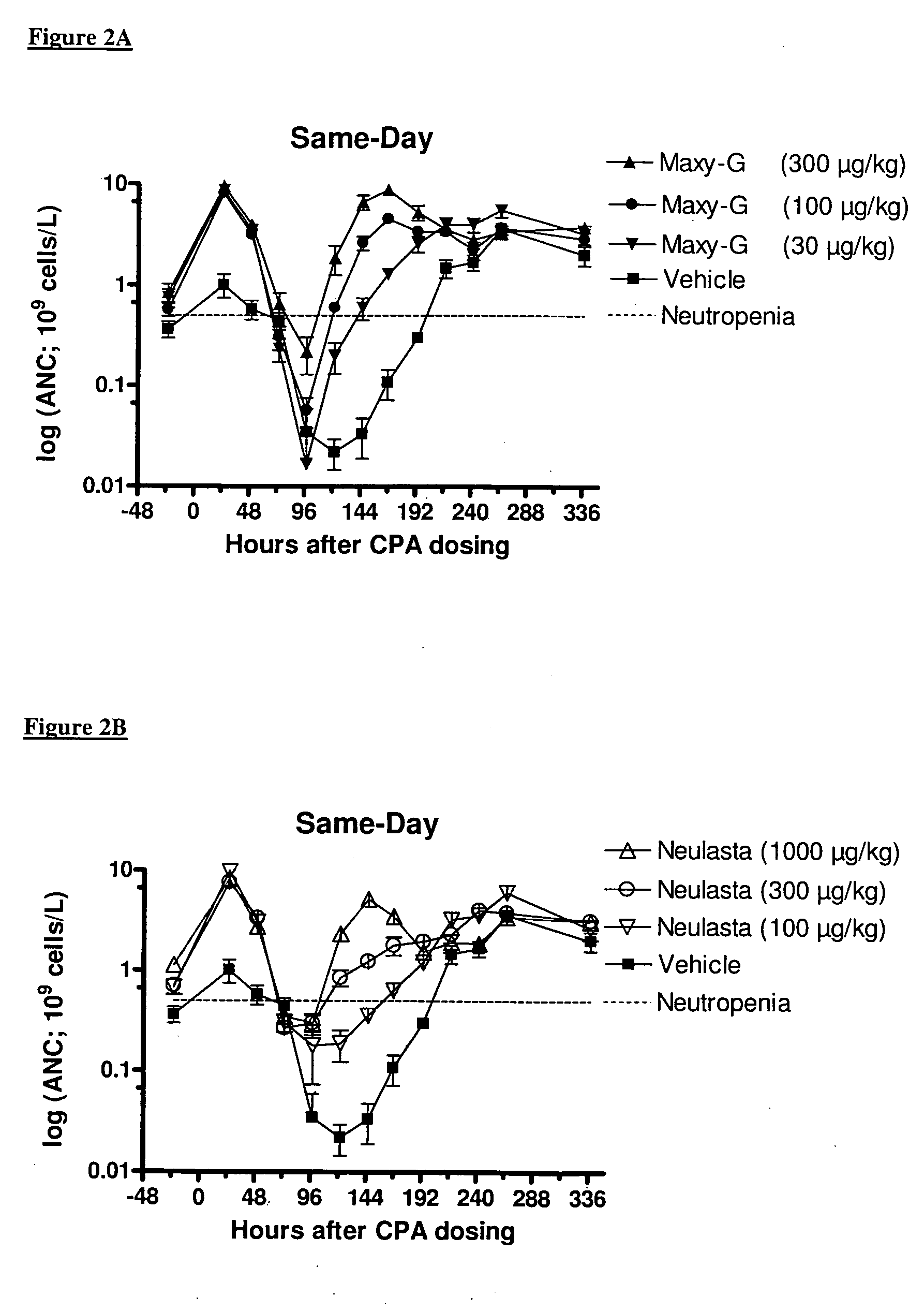
Method for the treatment of neutropenia by administration of a multi-pegylated granulocyte colony stimulating factor (G-CSF) variant
InactiveUS20090203601A1Reduce chemotherapy-induced neutropeniaTreating and preventing neutropeniaPeptide/protein ingredientsPharmaceutical non-active ingredientsPegylated granulocyte colony-stimulating factorGranulocytopenias
The invention relates to a method for treating or preventing neutropenia in a patient receiving chemotherapy by administering a multi-PEGylated granulocyte colony stimulating factor (G-CSF) variant on the same day that chemotherapy is administered.
Owner:MAXYGEN HLDG
Use of g-csf dimer in the treatment of neutropenia
InactiveUS20150147290A1Good clinical efficacyPeptide/protein ingredientsPharmaceutical non-active ingredientsMedicineHalf-life
This invention relates to a use of G-CSF dimer in the treatment of neutropenia. In particular, the recombinant human G-CSF of the present invention can enhance the differentiation and development of neutrophils in animal, and thus effectively reduce the severity of the severe neutropenia and shorten the time of severe neutropenia for the post-chemotherapy cancer patients. Serum half-life of G-CSF dimer of this invention is prolonged and the biological activity thereof is increased, providing a better effect in the treatment of neutropenia.
Owner:EVIVE BIOTECHNOLOGY (SHANGHAI) LTD
Acid-modified arabinogalactan protein composition
InactiveUS20030211077A1Antibacterial agentsOrganic active ingredientsDrug withdrawal symptomsArabinogalactan protein
An acid-modified arabinogalactan protein composition, having an arabinose-galactose ratio of less than 3.5:1 or less than 80% of the arabinose:galactose ratio of the arabinogalactan protein component of the composition prior to acid modification, prepared from Astragalus membranaceus, especially from the roots of Atragalus membranaceus, is capable of reconstitution into an aqueous intravenously injectable formulation; and is useful for stimulating hematopoiesis, inducing the proliferation or maturation of megakaryocytes, stimulating the production of IL-1beta, Il-6, TNF-alpha, IFN-gamma, GM-CSF, or G-CSF, stimulating the production or action of neutrophils, treating neutropenia, anemia, or thrombocytopenia, accelerating recovery from exposure (e.g. accidental or non-therapeutic exposure, as well as therapeutic exposure) to cytotoxic agents or radiation, treating cachexia, emesis, or drug withdrawal symptoms, or modifying biological responses or protecting hepatic cells in hepatitis B, in a mammal when intravenously administered to the mammal.
Owner:PHARMAGENESIS
Novel peptide exhibiting effect of releasing hematopoietic stem cells into blood and osteoporosis therapeutic effect and use thereof
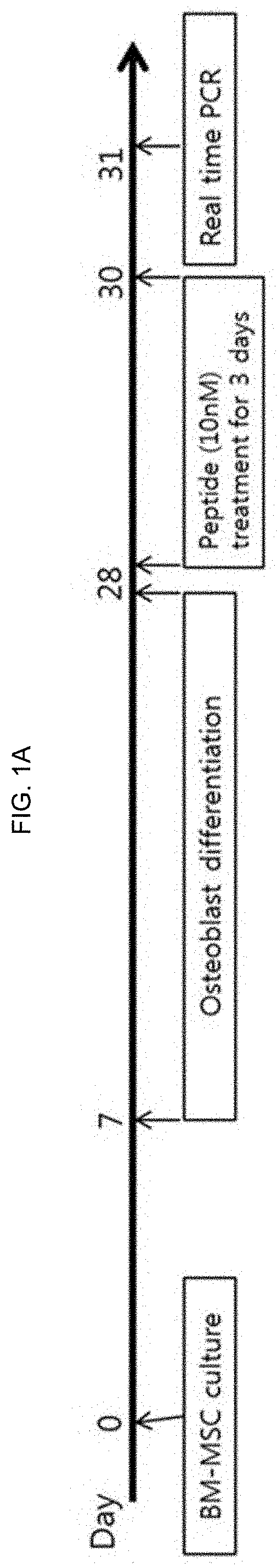
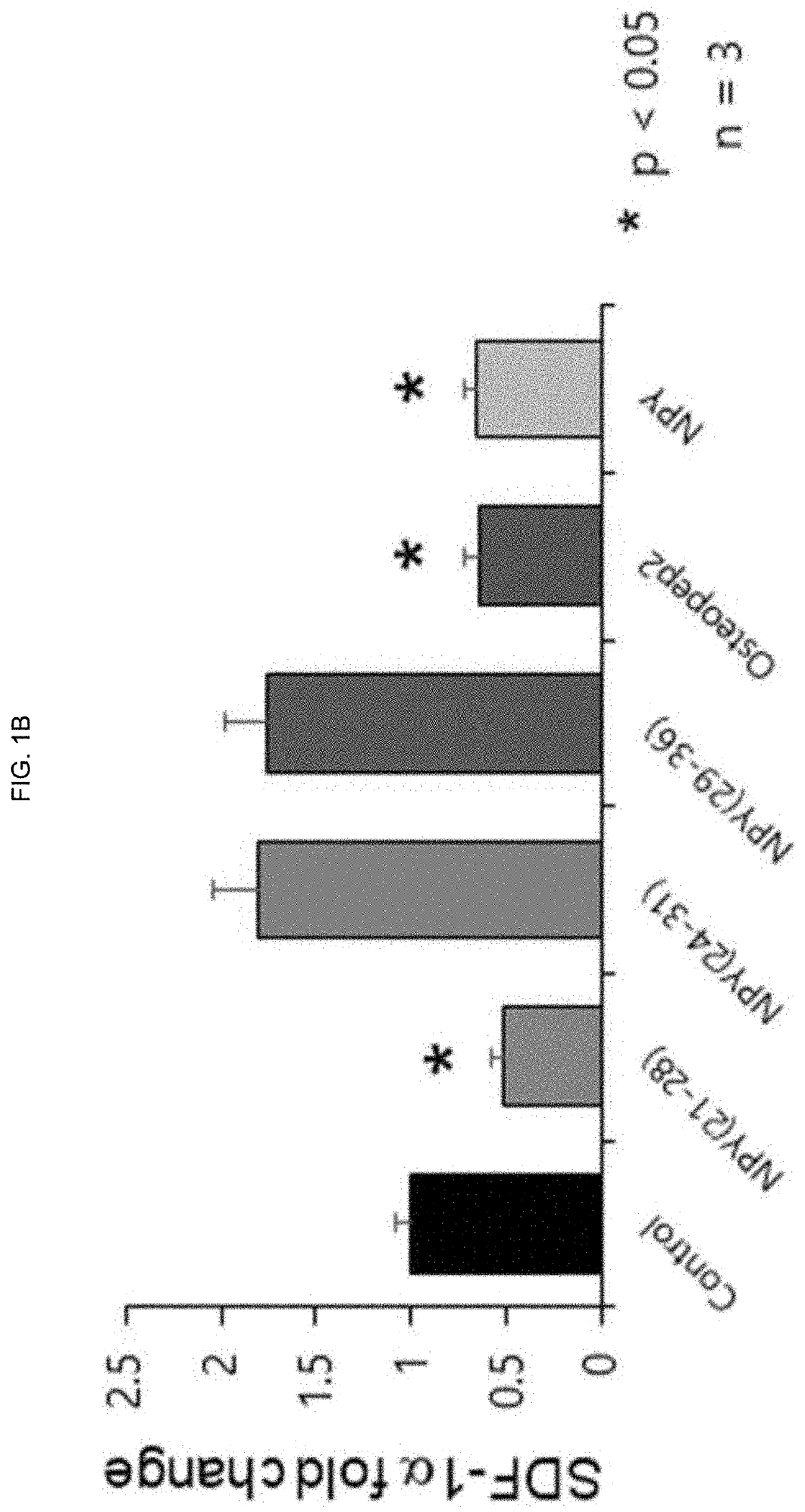
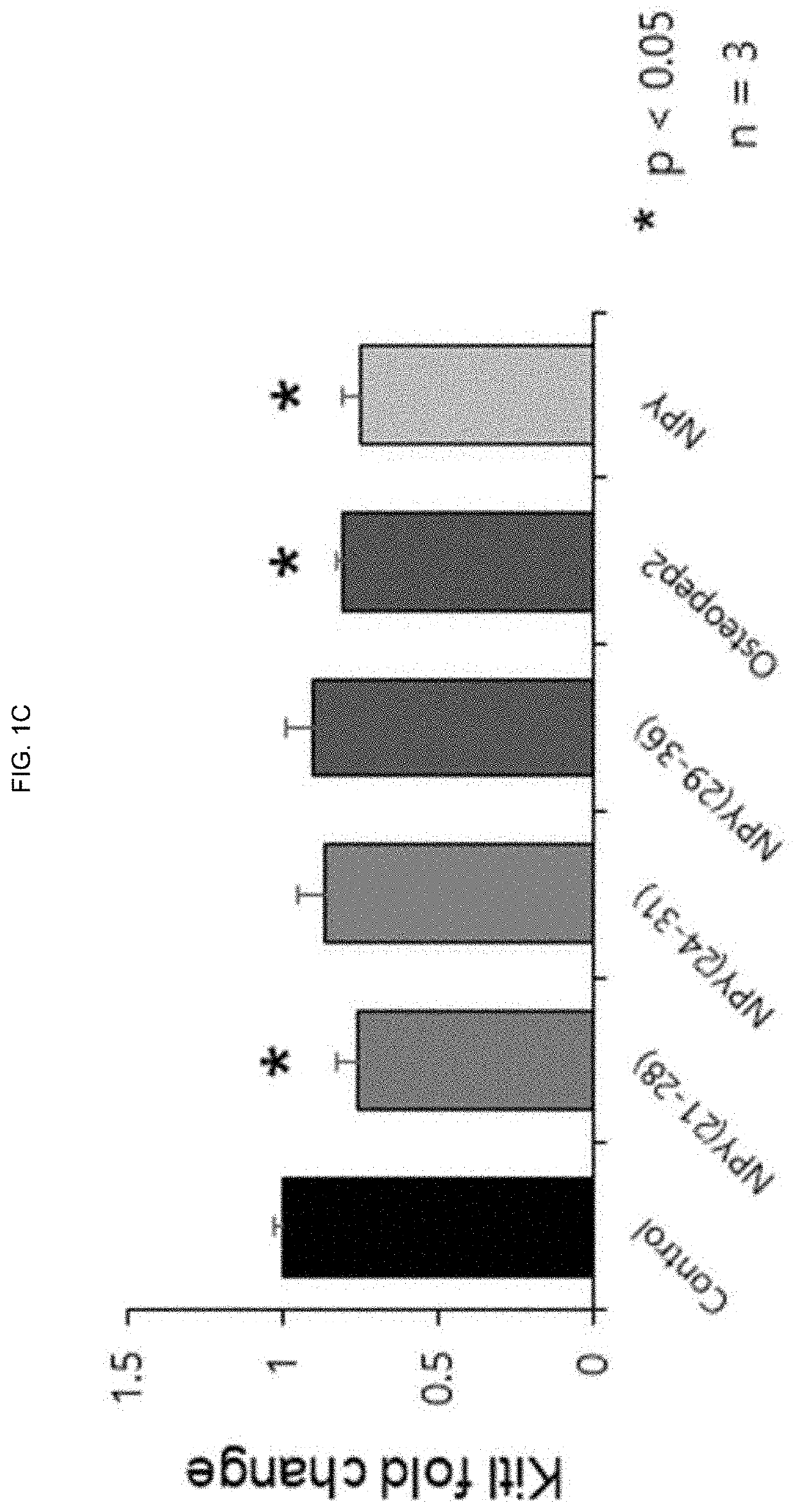
ActiveUS20200172592A1Reduce in quantityIncrease the number ofPeptide/protein ingredientsSkeletal disorderBone densityWhite blood cell
The present invention relates to a novel peptide exhibiting an effect of releasing myelopoiesis stem cells into blood and an osteoporosis therapeutic effect and use thereof and, in particularly, to a novel peptide consisting of an amino acid sequence of SEQ. ID.NO:1 which has effects of releasing hematopoietic stem cells into a bloodstream and decreasing osteoclast cells in bone narrow, and a pharmaceutical composition comprising the novel peptide as an active ingredient for preventing or treating neutropenia, anemia or osteoporosis. Because of side effects, the peptide of the present invention not only increases level of leukocytes, red blood cells and platelets in blood, but also alleviates a decrease in bone density, and therefore, can be very usefully used for the development of a prophylactic or therapeutic agent for neutropenia, anemia or osteoporosis.
Owner:KYUNGPOOK NAT UNIV IND ACADEMIC COOP FOUND
Compositions and methods for treating neutropenia
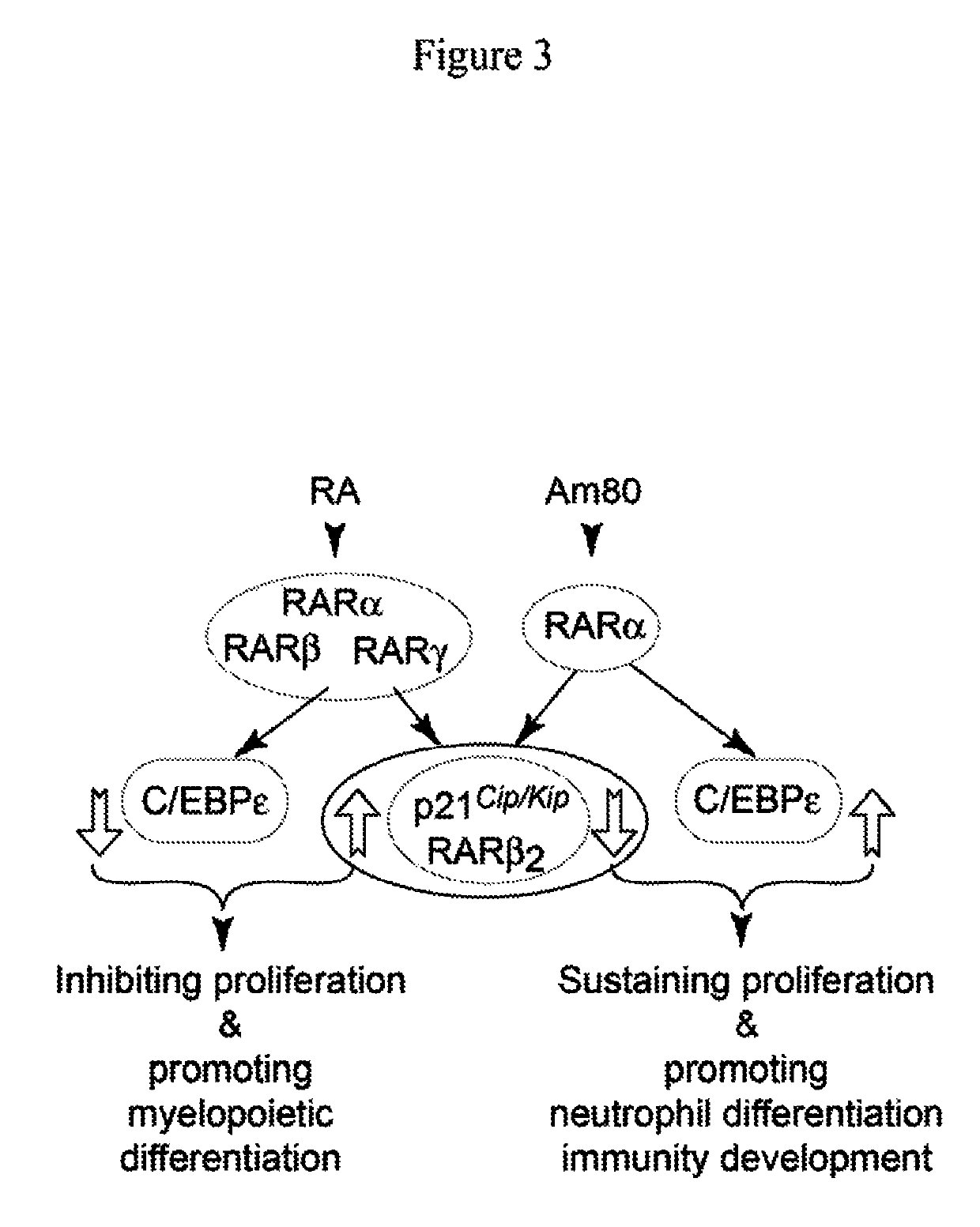
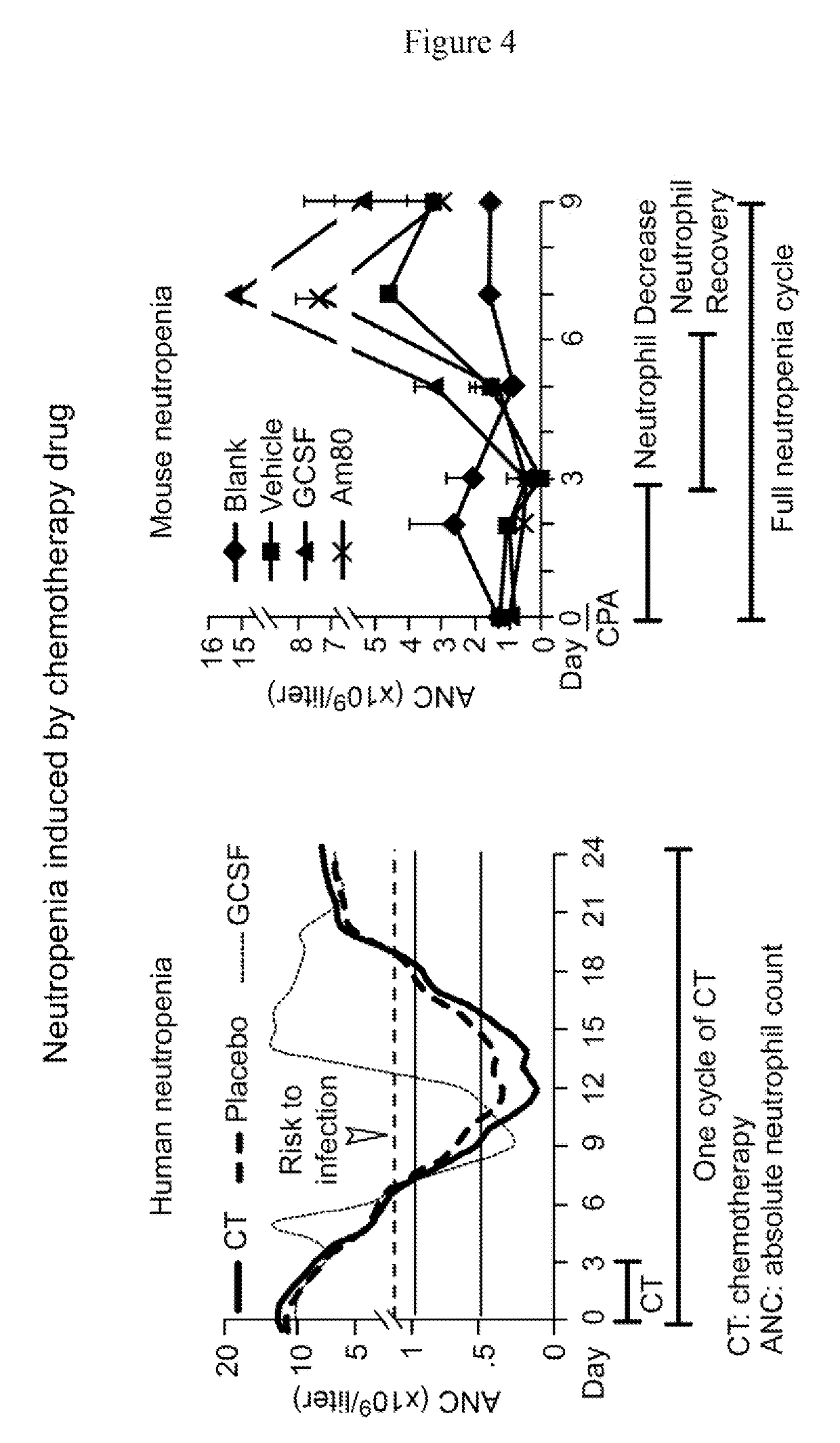
ActiveUS10286039B2Prevention and reduction of severityShorten the progressAntibacterial agentsPeptide/protein ingredientsRetinoidMedicine
The invention relates methods of using a retinoid agonist and a G-CSF or an analog thereof to treat, prevent, reduce the likelihood of having, reduce the severity of and / or slow the progression of a condition in a subject. The retinoid agonist and the G-CSF or the analog thereof may be provided in a single composition or in separate compositions. A therapeutically effective amount of the retinoid agonist and the G-CSF or the analog thereof may be administered to the subject concurrently or sequentially. Conditions treatable with the methods and compositions include but are not limited to various forms of neutropenia and microbial infections. The invention also relates to methods for determining the efficacy of the treatments described herein.
Owner:CHILDRENS HOSPITAL OF LOS ANGELES
A novel conjugate of granulocyte colony-stimulating factor (g-csf) with polyethylene glycol
InactiveCN103140499AHigh level of specific activityKinetic parameter improvementPeptide/protein ingredientsDepsipeptidesColony-stimulating factorGranulocytopenias
The present invention relates to pharmaceuticals and medicine, namely, to new physiologically active conjugates of granulocyte colony-stimulating factor (G-CSF) with the general formula (I) where: n - integers from 681 to 1 000; m - integer > 4; NaH-G-CSF - natural or recombinant polypetide, having the activity of G-CSF. The invention is also related to medicines containing the claimed conjugate of formula (I), pharmaceutical compositions, the use of conjugate of formula (I) for drugs and medicines with granulocyte colony stimulating factor as an active ingredient, approaches to prevent and / or treat neutropenia, the container that comprises pharmaceutical composition.
Owner:BIOCAD
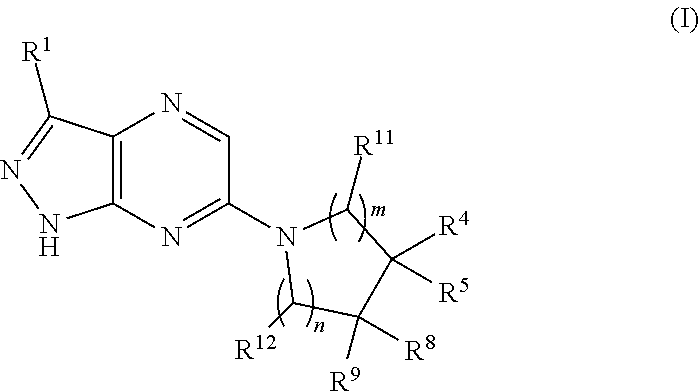
Method of treating inherited severe neutropenia
The invention is directed to a method of treating severe neutropenia, and in particular, cyclic neutropenia (CN) or severe congenital neutropenia (SCN), in a patient in need of such treatment comprising: administering a therapeutically effective amount of a compound of Formula (I) or a pharmaceutically acceptable salt thereof.
Owner:MERCK & CO INC +1
Mutant G-CSF fusion protein, and preparation and use thereof
ActiveUS8785597B2Improve biological activityRestoring the number of neutrophilic granulocytes more quicklyPeptide/protein ingredientsMicroorganismsHalf-lifeGranulocytopenias
The present invention relates to a mutant G-CSF fusion protein. The mutant G-CSF fusion protein is a fusion protein having the activity of stimulating the proliferation of neutrophilic granulocytes, and having a basic structure of G-CSF / carrier protein or carrier protein / G-CSF; wherein the G-CSF moiety comprises multipoint substitutions thus resulting in changes in biological activity and binding affinity. Compared with existing products, the mutant G-CSF fusion protein in the present invention has longer half-life and higher biological activity. Administration of the pharmaceutical preparation containing this mutant G-CSF fusion protein could be used in the treating neutropenia.
Owner:JIANGSU T MAB BIOPHARMA
Method of treating inherited severe neutropenia
The invention is directed to a method of treating severe neutropenia, and in particular, cyclic neutropenia (CN) or severe congenital neutropenia (SCN), in a patient in need of such treatment comprising: administering a therapeutically effective amount of a compound of Formula (I) or a pharmaceutically acceptable salt thereof.
Owner:MERCK & CO INC +1
Chinese traditional medicinal composition and its preparation process and usage
ActiveCN1593594AHas the effect of regulating the immune systemReduce oxygen consumptionAntiviralsUnknown materialsMedicinal herbsCurative effect
The invention discloses a medicinal composition which is prepared from he following Chinese medicinal herbs (by weight ratio), notoginseng 10-30%, ginseng 10-10%, ophiopogon root 40-80%. The invention also discloses the process for preparing the pharmaceutical composition and use thereof.
Owner:JIANGSU KANION PHARMA CO LTD
Method and kit for estimating side effect by paclitaxel therapy
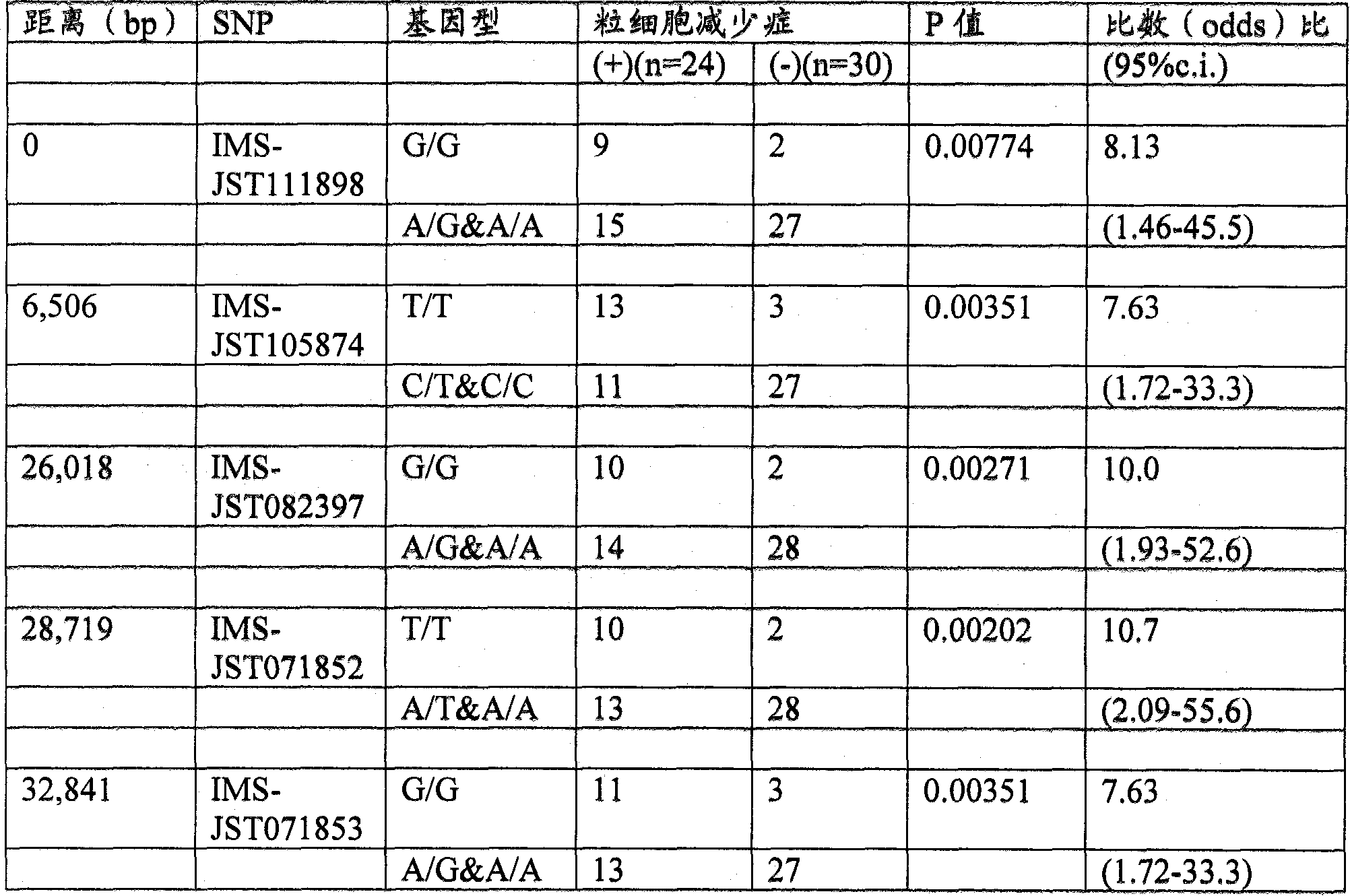
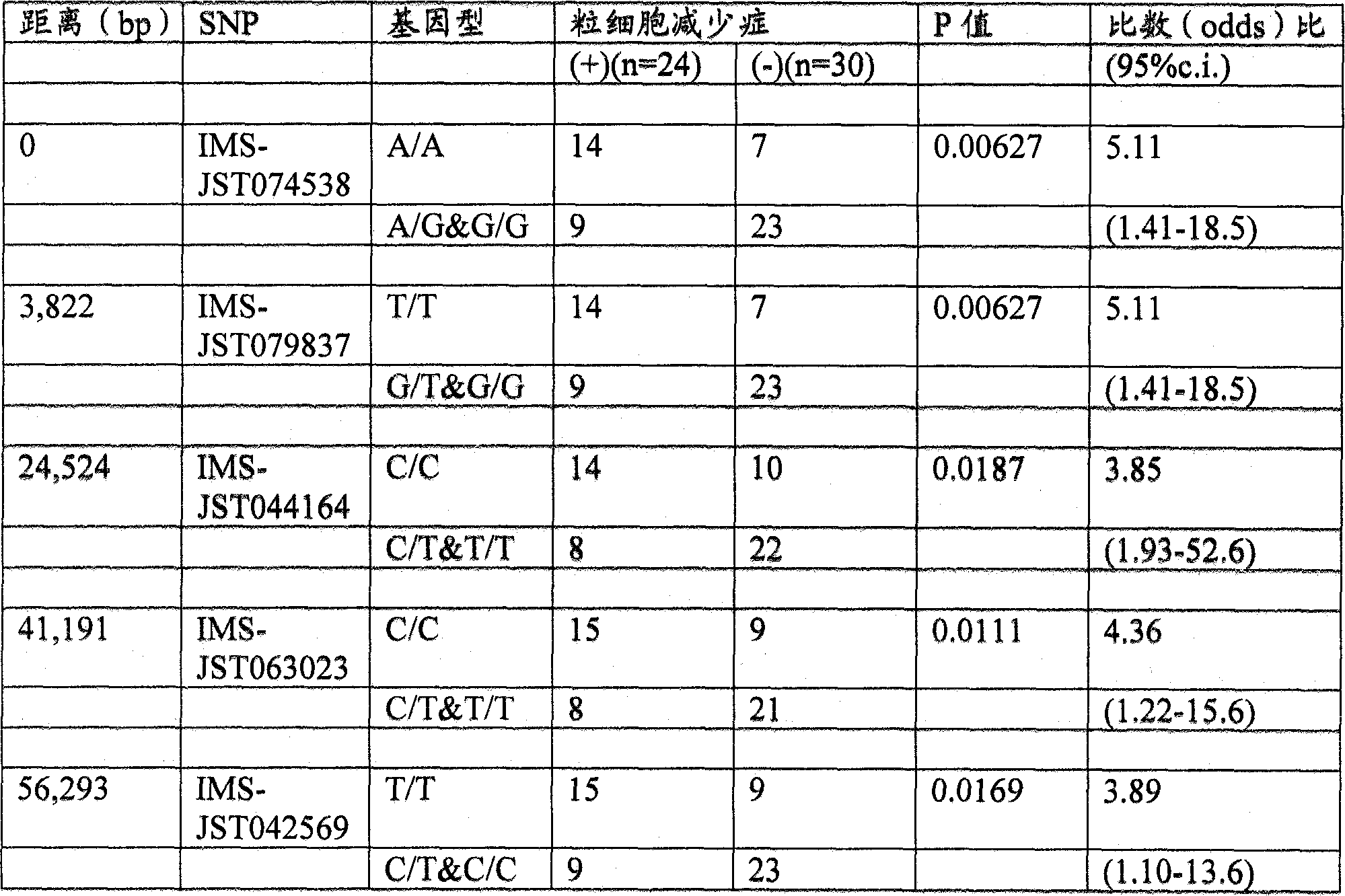
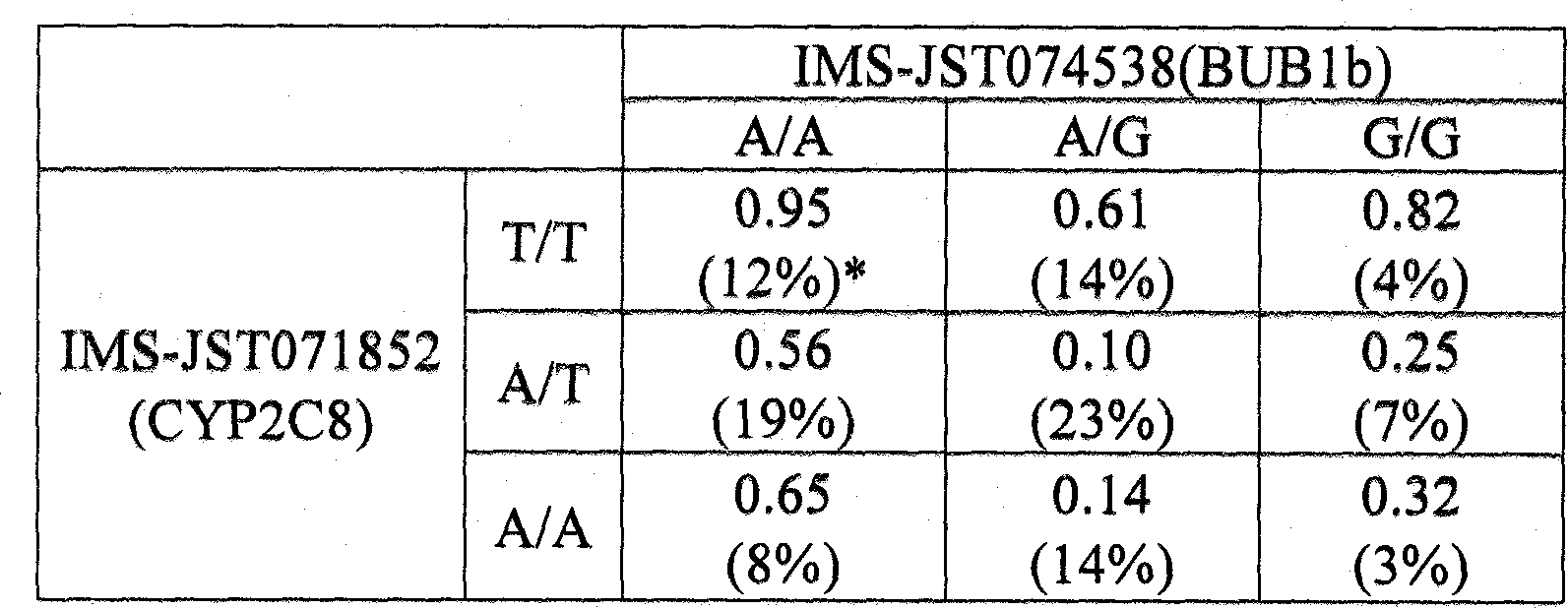
It is intended to disclose a method for risk estimation of the onset of granulocytopenia in a subject caused by paclitaxel treatment. This method comprises identifying gene polymorphisms in genes isolated from the subject, i.e., at five SNPs (IMS-JST111898 (SEQ ID NO:1), IMS-JST105874 (SEQ ID NO:2), IMS-JST082397 (SEQ ID NO:3), IMS-JST071852 (SEQ ID NO:4) and IMS-JST071853 (SEQ ID NO:5)) in CYP2C8 gene, and at five SNPs (IMS-JST074538 (SEQ ID NO:6), IMS-JST079837 (SEQ ID NO:7), IMS-JST044164 (SEQ ID NO:8), IMS-JST063023 (SEQ ID NO:9) and IMS-JST042569 (SEQ ID NO:10)) in BUB1b gene. Also, a kit containing a reagent to be used in this method is disclosed.
Owner:JAPANESE FOUND FOR CANCER RES
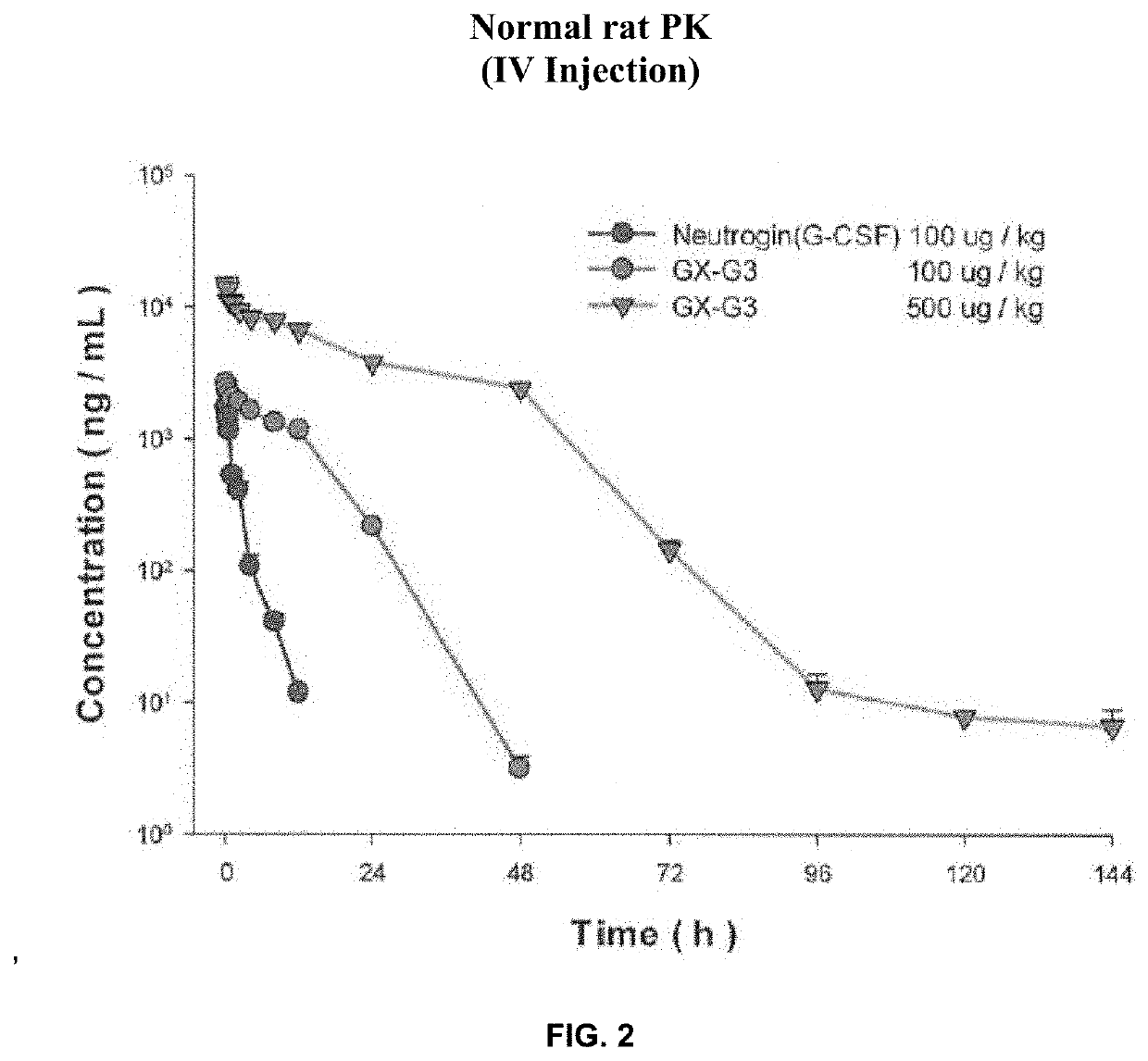
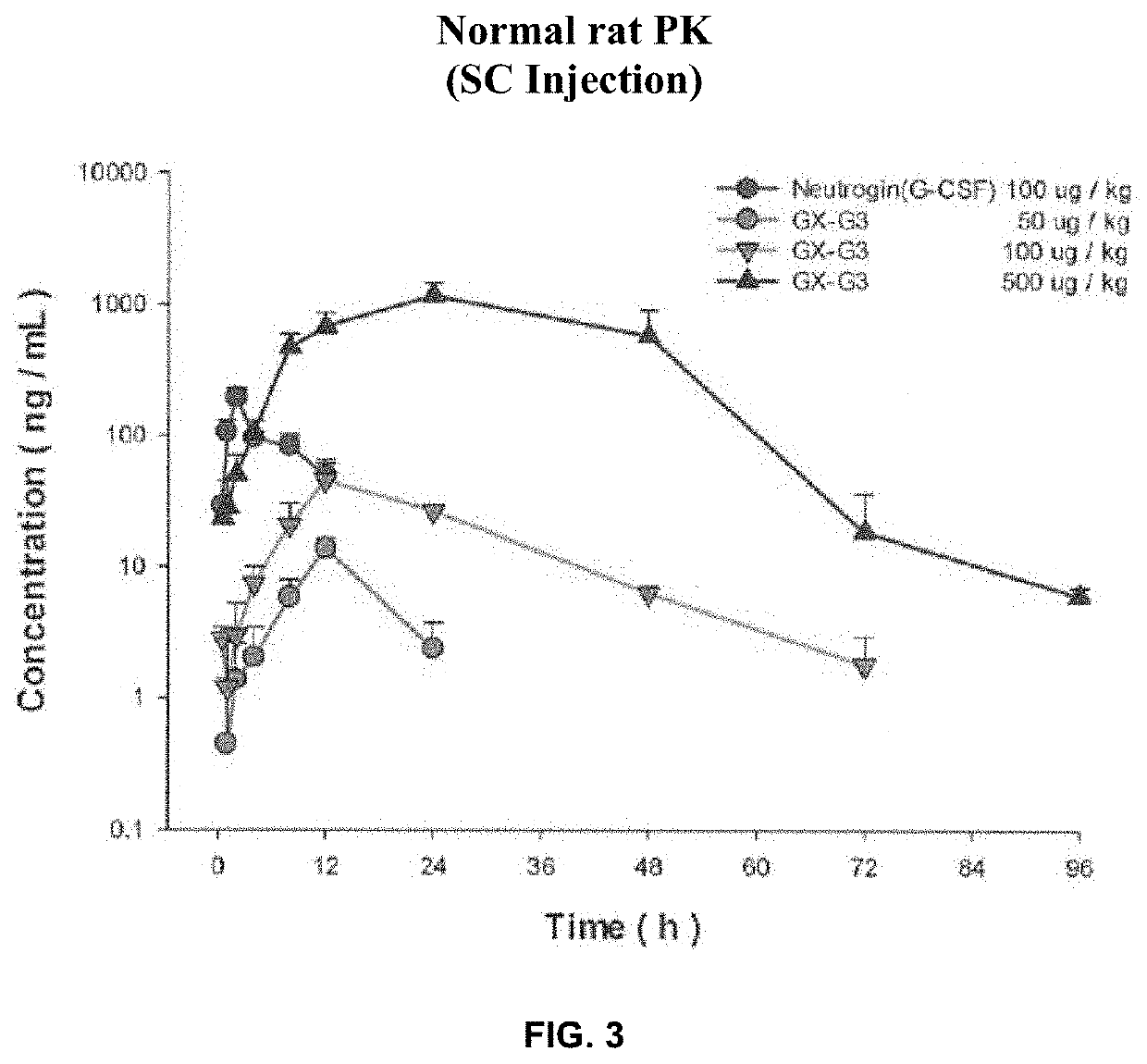
Long-acting g-csf for preventing neutropenia or reducing duration of neutropenia
PendingUS20200255842A1Shorten the construction periodPreventing neutropeniaPeptide/protein ingredientsAntibody mimetics/scaffoldsGranulocytopeniasNeutrophil granulocyte
A method for preventing neutropenia or reducing the duration of neutropenia in a patient by administering therapeutically effective amount of a human hybrid (hy) Fc fused granulocyte colony stimulating factor (G-CSF) developed as next-generation G-CSF.
Owner:ILKOGEN ILAC SANAYI VE TICARET AS
Methods for treating refractory infections in neutropenic individuals
The invention is based, in part, on the discovery of a novel cell-based immunotherapy that can recapitulate neutrophil functions in neutropenic individuals afflicted with a microbial infection. The therapeutic methods of the invention are broadly applicable to treat any infection in a neutropenic individual, including infections caused by bacteria, fungi, protozoa, and viruses. The methods of the invention represent a practical, rapid cell-based immunotherapy for refractory infections comprising compositions of activated, irradiated HL-60 cells.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com