Nitrogen-terminal fixed-point coupling method for colony stimulating factor of column chromatography granulocyte and coupled product
A colony-stimulating factor and granulocyte technology, which is used in PEGylated recombinant human granulocyte colony-stimulating factor conjugated compounds, the application of long-acting preparations of protein compounds, and the use of column chromatography PEGylated protein nitrogen-terminal point-specific couplings. It can solve problems affecting application value and significance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0173] Example 1 Expression and preparation of rhG-CSF and rhG-CSFm
[0174] Expression of rhG-CSF and rhG-CSFm:
[0175] Fresh human bone marrow cells were cultured and activated with pokeweed kinogen (Sigma, USA). Human bone marrow cell mRNA was extracted, and the hG-CSF gene was amplified by RT-PCR method, which was confirmed to be hG-CSF gene through sequencing (completed by Beijing Boshang Biotechnology Co., Ltd.), and its cDNA sequence is shown in SEQID NO.2. The amino acid sequence is shown in SEQID NO.1. DNA sequence analysis of hG-CSF gene was analyzed by DNA Strider software; PCR (polymerase chain reaction)) and site-directed mutagenesis were used to modify the human natural G-CSF gene sequence to obtain a new modified human granulocyte colony-stimulating factor ( rhG-CSF) gene coding sequence, its nucleotide sequence is shown in SEQID NO.3, this gene is constructed on the pMD18-T vector, and the vector is transformed into Top10 bacterial strain and preserved.
[...
Embodiment 2
[0198] Example 2 Preparation of PEG-rhG-CSF by site-directed coupling of PEG-propionaldehyde and rhG-CSF N-terminus
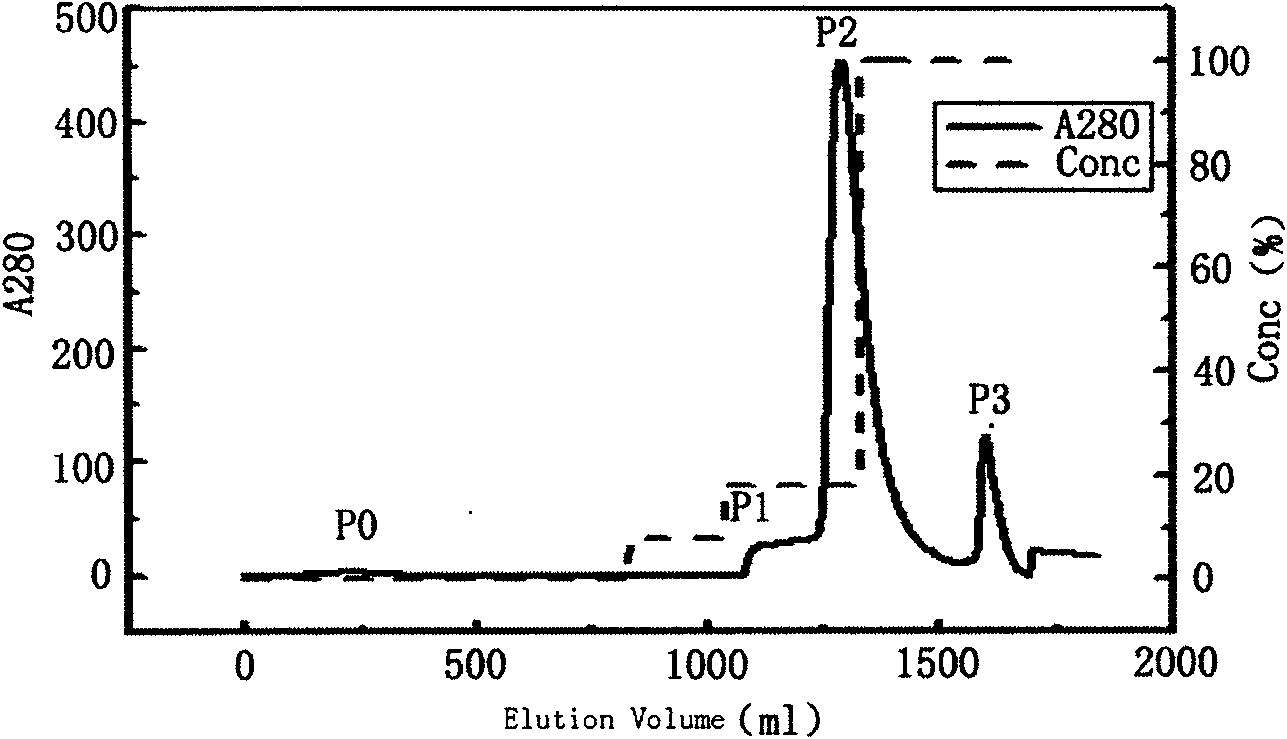
[0199] Load MacroCap SP strong cation exchange chromatographic column (2.6cmX20cm), and connect it to AKTAExplorer 100 liquid chromatography system (Amersham Bioscience, Sweden), use 20mM sodium acetate buffer pH 5.5 (buffer solution A) and 0.005% poly Sorbitol ester 80 500ml fully equilibrates the chromatographic column; using a feed pump, add 0.5mg / ml 1500ml of rhG-CSF stock solution to the chromatographic column at a flow rate of 10ml / min; Solution A washes the column to remove substances that do not electrostatically adsorb with the chromatographic medium; at a flow rate of 3ml / min, add 5mg / ml 30kDa PEG-propionaldehyde (PEG-propionaldehyde) and 0.1mg / mlNaBH 3 CN solution 1500ml, continuous sample loading for about 240 minutes; the pH of the reaction system is 5.5; then wash the column with about 500ml buffer A at a flow rate of 15ml / min to remove substances...
Embodiment 3
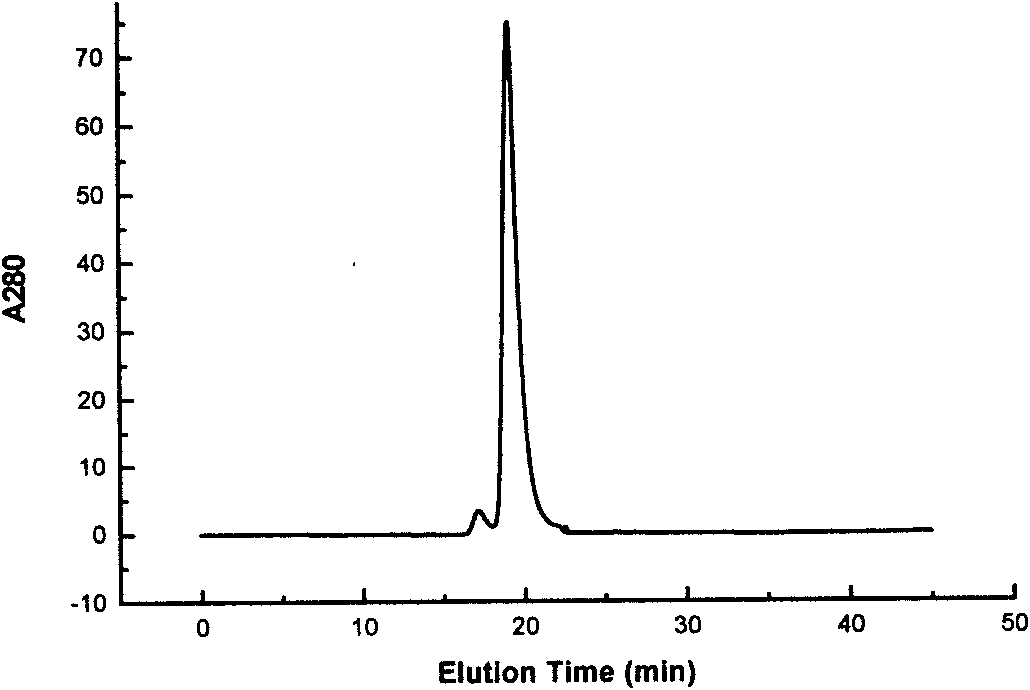
[0203] Example 3 Analysis of the Coupling Site of PEG-ALD and rh-CSF Using Trypsin Digestion Peptide Mapping by Liquid-MS
[0204] 1) Measurement method
[0205] Select liquid chromatograph Agilent 1100 and mass spectrometer LCQ Deca XP MS to analyze, and specific method is as follows:
[0206] Liquid chromatograph operating conditions:
[0207] The mobile phase is: A: 0.1% A: 0.1% TFA in water, B: 0.1% TFA in ACN;
[0208] Chromatographic conditions: the chromatographic column is Zorbax SBC18 (2.1 × 150mm, Agilent), the flow rate is 0.2ml / ml, the gradient is 0-60min, 5%B-60B, 60-120min, 60%B-90%B, and the detection wavelength is 214nm;
[0209] Mass spectrometer operating conditions:
[0210] Sheath gas: 60arb; Aux gas: 0; Spray voltage: 4.5kV; Detection method: Triple play; Primary mass spectrometry: Mass range: m / z300-2000; Zoom scan: Data dependent mode; Data processing software: Bioworks3.1; mass spectrometry data analysis software Turbosequest3.1.
[0211] The enzy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com