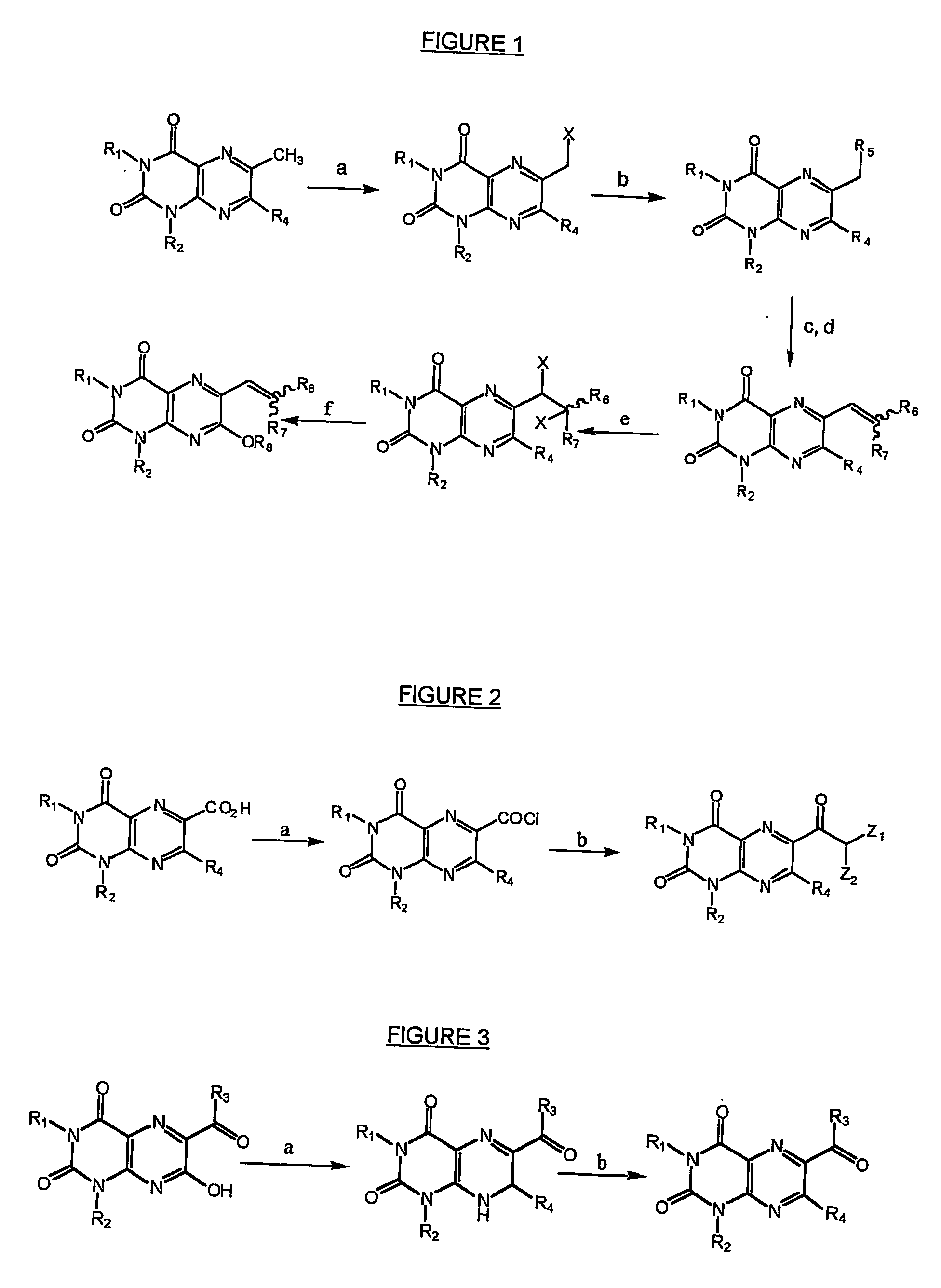
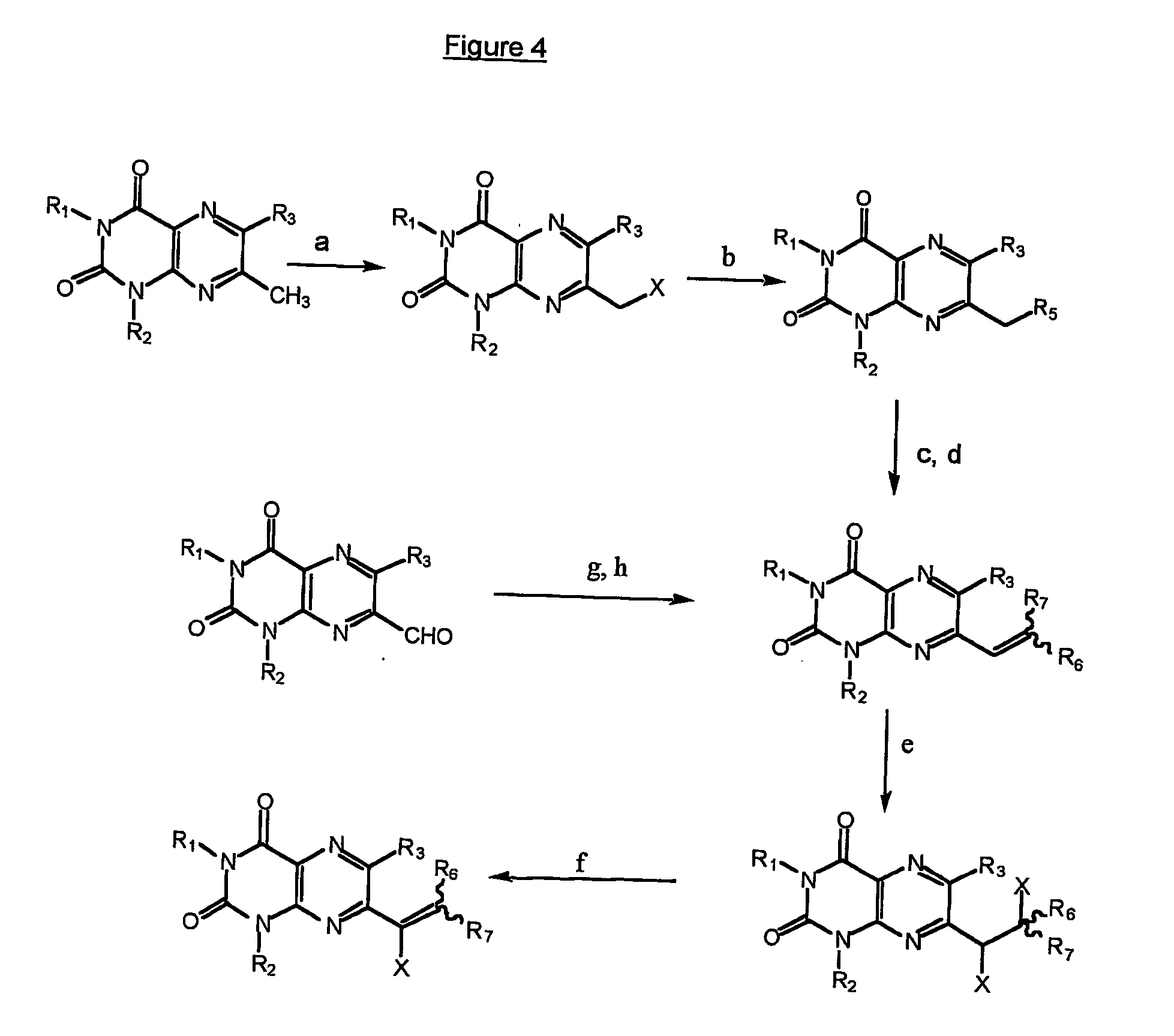
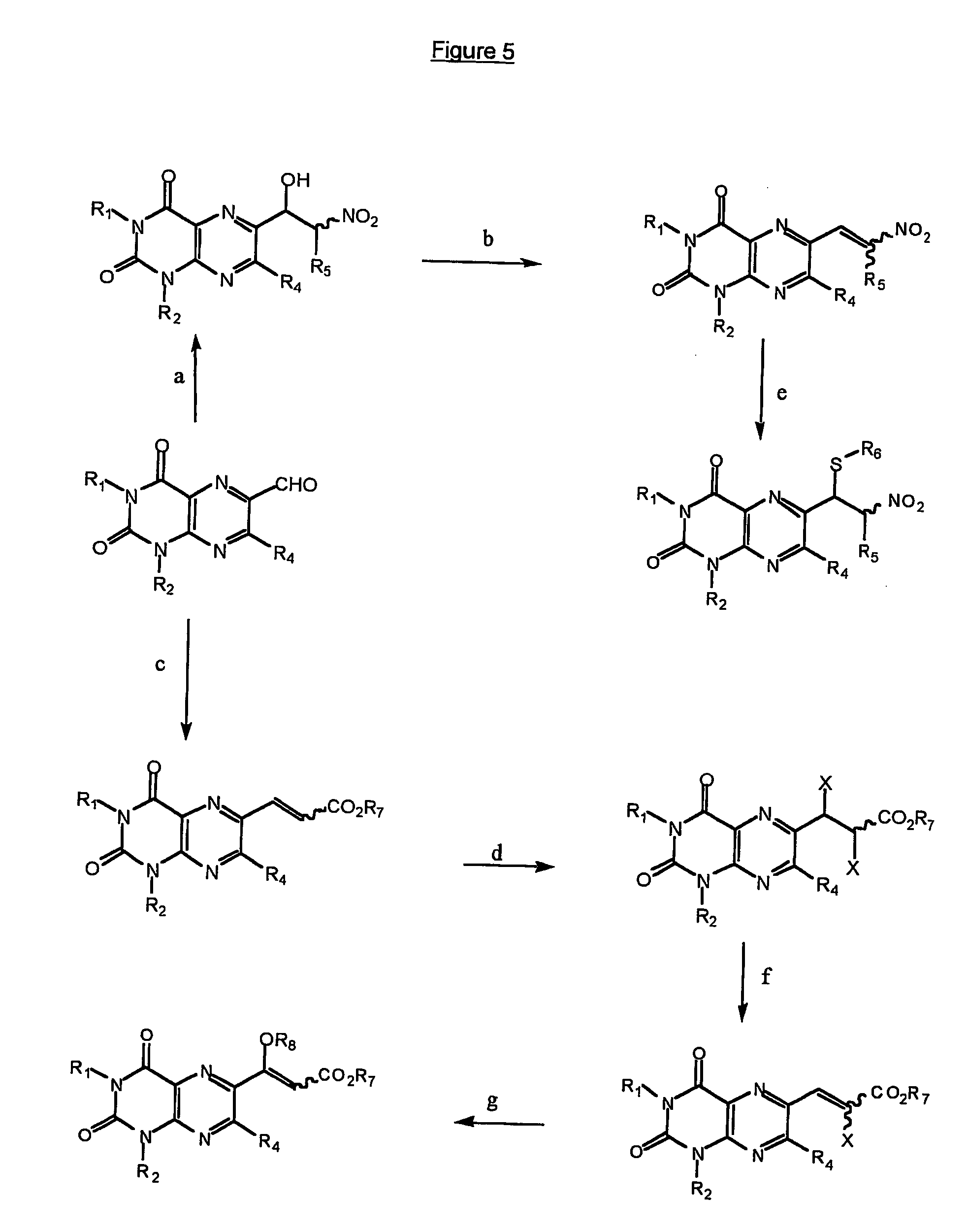
Immunosuppresive effects of pteridine derivatives
a technology of pteridine and derivatives, which is applied in the direction of biocide, plant growth regulators, cyclic peptide ingredients, etc., can solve the problems of high turn-over rate, marrow depression and liver damage, and severe toxic effects on normal cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1 to 5
Preparation of Tri- and Tetra-Substituted 1,3-dimethyl-lumazines
[0215] The following tri- and tetra-substituted 1,3-dimethyl-lumazines were prepared according to methods and procedures described hereinbefore by reference to FIGS. 6 and 7: [0216] 6-[2-(p-trifluoromethylphenyl)ethenyl]-1,3-dimethyl-lumazine (example 1), [0217] 6-[2-(p-trifluoromethylphenyl)ethenyl]-1,3-dimethyl-lumazine (example 2), [0218] 6-[2-phenylethenyl]-1,3-dimethyl-lumazine (example 3), [0219] 6-[2-(p-trifluoromethoxyphenyl)ethenyl]-1,3-dimethyl-lumazine (example 4), and [0220] 6-cyano-7-ethylmercaptoacetate-1,3-dimethyl-lumazine (example 5).
example 6
Preparation of 3-methyl-6-phenyl-7-chloro-lumazine
[0221] 3-methyl-6-phenyl-7-chloro-lumazine was prepared according to the general methods described hereinbefore by reference to FIG. 6.
examples 7 to 20
Preparation of 1,6-Disubstituted 7-hydroxylumazines
[0222] In a first step, trisubstituted lumazines having the general formula (I) wherein R1 is hydrogen, R4 is hydroxyl, and R2 and R3 are as indicated in table 1 below for each of examples 7-13, were prepared according to the general methods described hereinbefore by reference to FIG. 6, in particular steps (d) and (e) thereof, and more specifically according to the following procedure:
[0223] To a suspension of a 5,6-diamino-1-substituted uracil hydrate (10 mmole) in 200 ml water, ethyl substituted benzoylformate (12.5 mmole) was added. The resulting mixture was heated under reflux for 40 minutes. After cooling to room temperature, the precipitate was collected to yield a crude product which was re-crystallized from a MeOH / water (1 / 1) mixture to yield yellowish crystals or powder of the desired 7-hydroxyl tri-substituted lumazine.
TABLE 1R2R3Examplemethylphenyl7methylp-methoxyphenyl8methylp-toluyl9phenylphenyl10benzylphenyl11meth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com