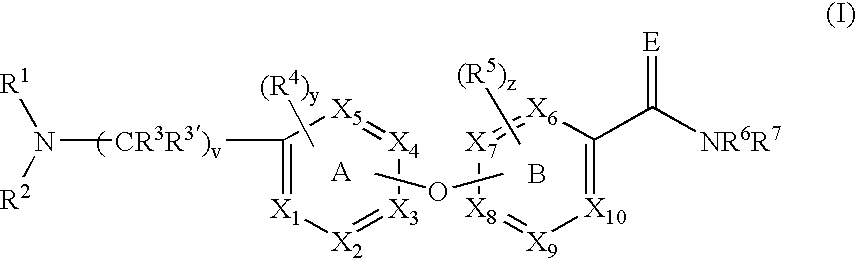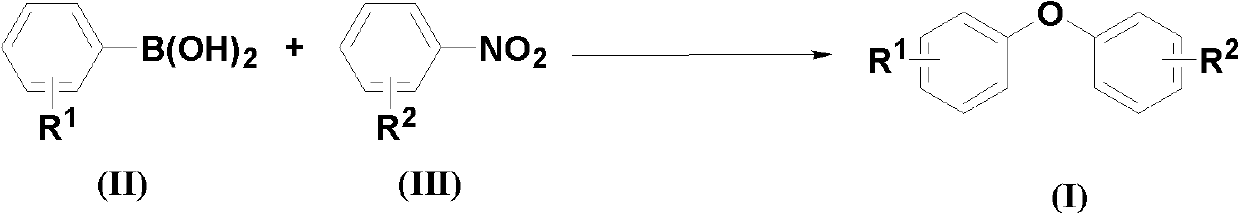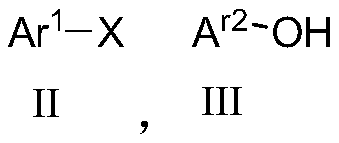Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
73 results about "Diaryl ether" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
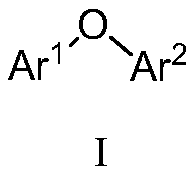
Diaryl ethers are readily synthesized in high yield at room temperature through the copper(II)-promoted coupling of arylboronic acids and phenols. The reaction is tolerant of a wide range of substituents on both coupling partners. These reaction conditions permit the racemization-free arylation of phenolic amino acids.
Diaryl ethers as opioid receptor antagonist
ActiveUS20060217372A1Overall light weightReduce weightSenses disorderNervous disorderExomer complexEnantiomer
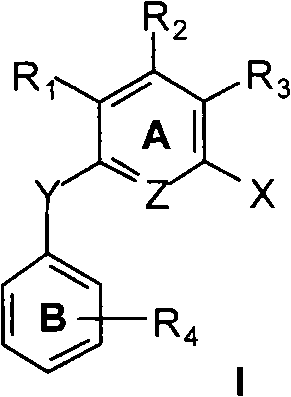
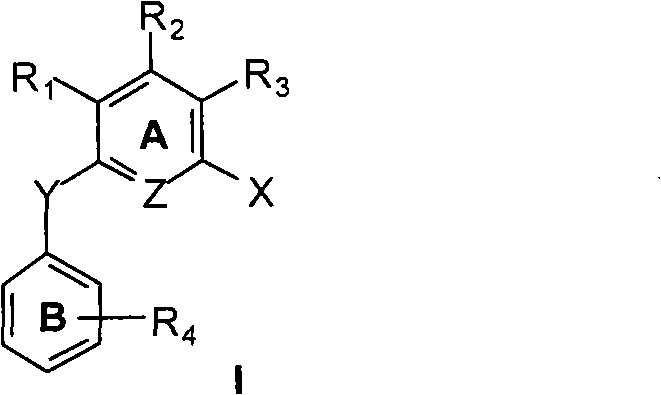
A compound of the formula (I) wherein the variables X1 to X10, R1 to R7 including R3′, E, v, y, z, A and B are as described, or a pharmaceutically acceptable salt, solvate, enantiomer, racemate, diastereomer or mixtures thereof, useful for the treatment, prevention or amelioration of obesity and Related Diseases is disclosed.
Owner:ELI LILLY & CO
Diaryl ether derivative, addition salt thereof, and immunosuppressant
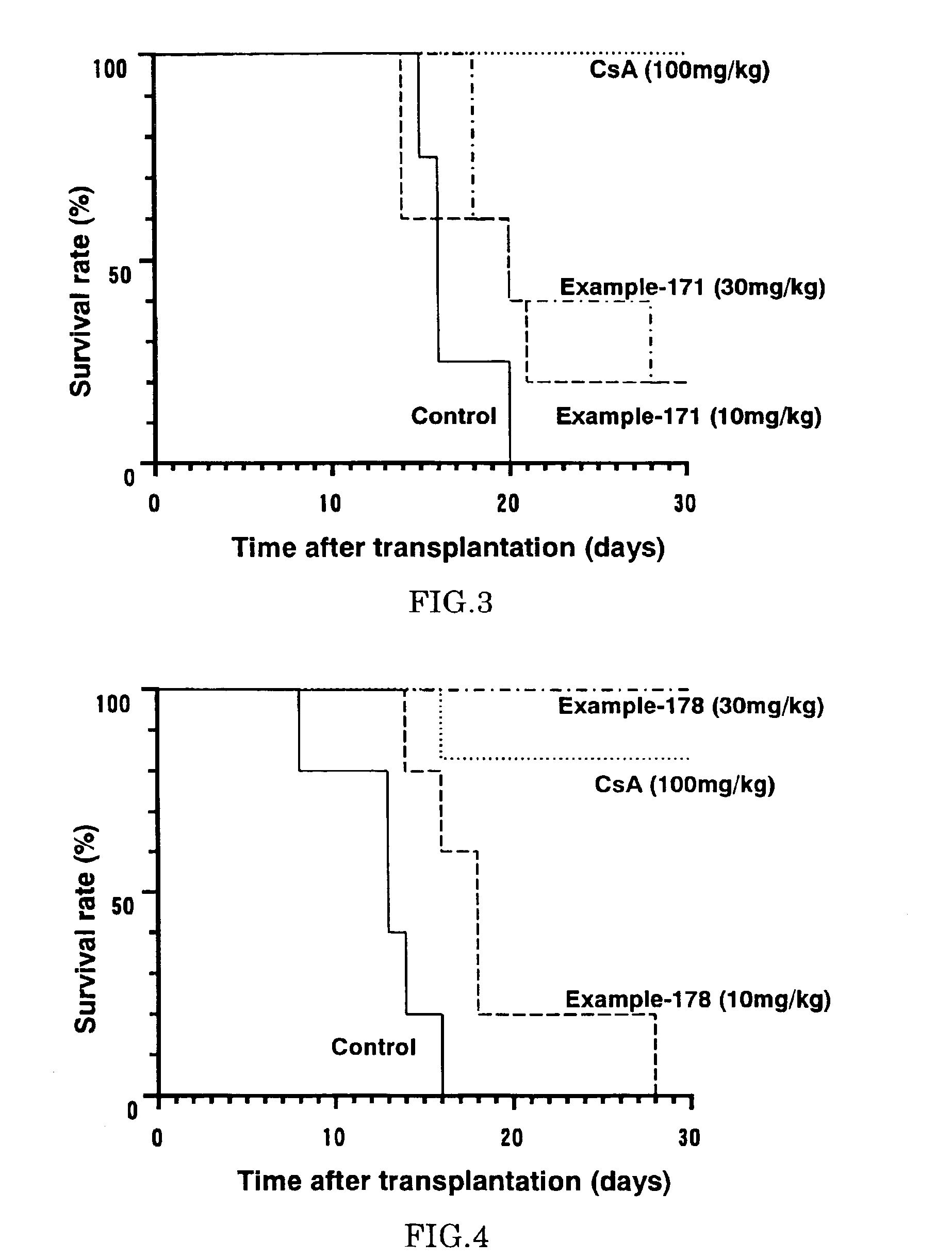
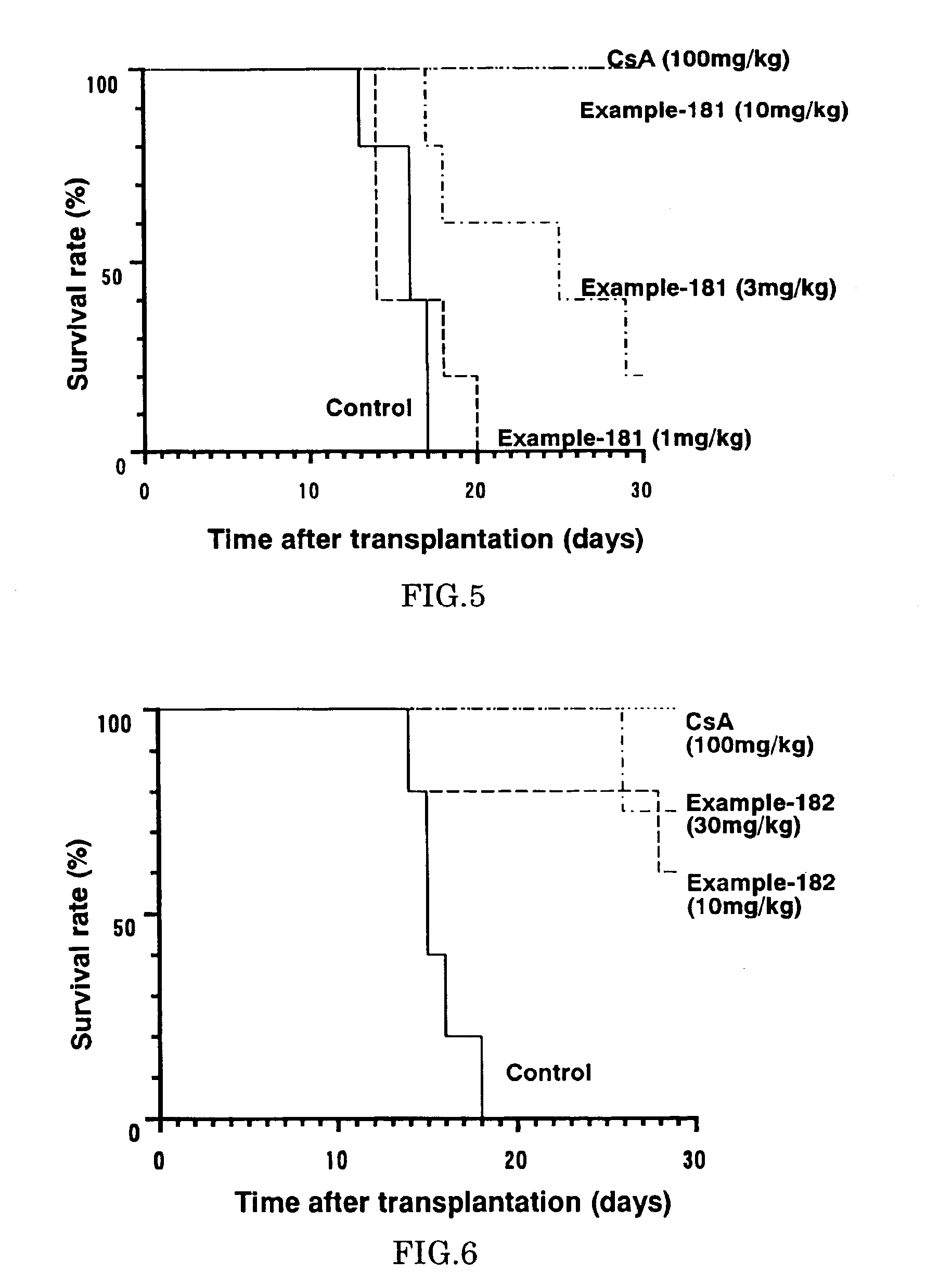
The present invention provides diaryl ether derivatives that exhibit significant immunosuppressive effects with less side effects.The diaryl derivatives of the present invention are represented by the following general formula (1): one example is 2-amino-2-[4-(3-benzyloxyphenoxy)-2-chlorophenyl]propyl-1,3-propanediol.
Owner:KYORIN PHARMA CO LTD
Diaryl ethers as opioid receptor antagonist
A compound of the formula (I) wherein the variables X1 to X10, R1 to R7 including R3′, E, v, y, z, A and B are as described, or a pharmaceutically acceptable salt, solvate, enantiomer, racemate, diastereomer or mixtures thereof, useful for the treatment, prevention or amelioration of obesity and Related Diseases is disclosed
Owner:ELI LILLY & CO
Effective Use Method Of Medicaments And Method Of Preventing Expression Of Side Effect
ActiveUS20080032923A1Reduce circulationImprove immunosuppressionOrganic active ingredientsSenses disorderArylSide effect
A medicine which effectively functions as an immunosuppressant or anti-inflammatory agent and is effective in diminishing the occurrence of side effects. The medicine comprises a combination of: a diaryl sulfide or diaryl ether compound having a 2-amino-1,3-propanediol structure and having the function of diminishing lymphocytes circulating through the periphery; and an immunosuppressant and / or an anti-inflammatory agent.
Owner:PRIOTHERA LTD
N-substituted arene aniline / polysubstituted diaryl ether compound, preparation and anti-tumor use thereof
The invention relates to formula (I) N-substituted aromatic hydrocarbon phenyl amines and polysubstitution diaryl ether compounds or medicinal salt thereof, wherein, definitions of X, Y, Z, R1-R4 are shown in a claim, and also relates to a preparing method thereof, a drug combination containing the compounds and the application to anti cancer drug preparation thereof.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Method for synthesizing asymmetric diaryl ether derivative
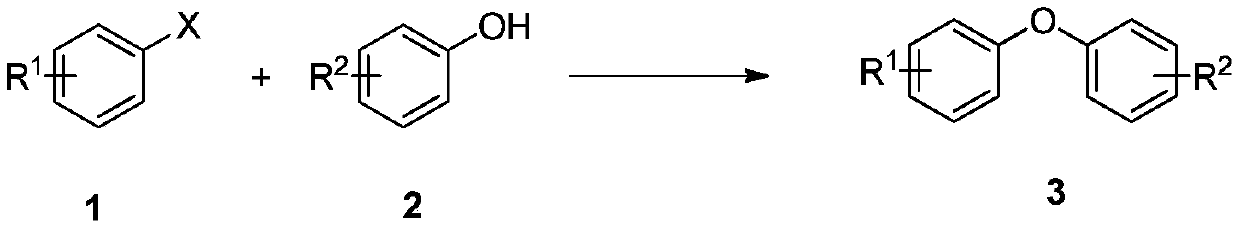
The invention discloses a method for synthesizing asymmetric diaryl ether derivative with a structure shown in a structural formula (I), comprising the following steps: making arylboronic acid and nitrobenzene compound which are raw materials to fully react in an inert organic solvent in the presence of a copper catalyst, an additive and an alkaline compound at 30-150 DEG C, wherein the arylboronic acid has the structural formula (II), and the nitrobenzene compound has the structural formula (III); and postprocessing, separating and purifying a reaction solution after the reaction finishes to obtain the asymmetric diaryl ether derivative. The method for synthesizing the asymmetric diaryl ether derivative has the advantages of mild reaction condition, high reaction yield, good selectivity and low-cost generation cost.
Owner:WENZHOU UNIVERSITY
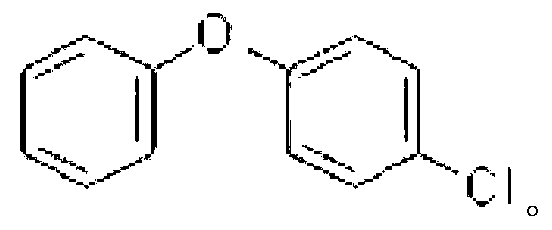
Preparation method for 3,4'dichloro diaryl ether
InactiveCN102516044ARaise the ratioSimple wayOrganic chemistryOrganic compound preparationDiphenyl etherPtru catalyst
A preparation method for 3,4'dichloro diaryl ether is disclosed, which comprises the following steps of: (1) preparation for potassium alkoxide or sodium alkoxide; (2) preparation for potassium p-chlorophenolate; (3) preparation for active cuprous chloride; and (4) preparation for 3,4'dichloro diaryl ether. The preparation method has the following advantages that: side reactions are greatly reduced, reaction speed is fast, the pollution caused by the volatilization of benzene solvents is reduced, the utilization rate of equipment and the like are increased, the quality of product is improved, as well as yield and chilling efficiency are increased by using p-chlorophenol waterless salifying process, using newly-prepared catalyst, optimizing the ratio and pouring method of materials, and protecting by inert gas.
Owner:HUBEI XINGHUO CHEM
Method for synthesizing diaryl ether by using amino acid as additive
InactiveCN1462735ALow priceEasy to separateCarboxylic acid nitrile preparationOrganic compound preparationNitrogenDiaryl ether
A process using amino acids as additive for synthesizing biarylether features use of amino acid as additive and Cu salt as catalyst. The said amino acid may be the monosubstituted (or bisubstituted)-on-nitrogen alpha-amino acid or beta-amino acid. Its advantages are very gentle reaction, condition and high output rate.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
Diaryl ether beta2 adrenergic receptor agonists
The invention provides novel β2 adrenergic receptor agonist compounds. The invention also provides pharmaceutical compositions comprising such compounds, methods of using such compounds to treat diseases associated with β2 adrenergic receptor activity, and processes and intermediates useful for preparing such compounds.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
1-3 generation arylene ether dendritic phthalocyanine complex and polymer nano-particle thereof
InactiveCN101580506AGood water solubilityHigh photosensitivityOrganic active ingredientsOrganic chemistryDendrimerEnd-group
The invention discloses a 1-3 generation arylene ether-substituted dendritic phthalocyanine zinc complex, a preparation method and an application thereof. The method comprises the following steps of adopting a Frechet synthesis method to synthesize the first to third generation alcohol molecules which take the 1-3 generation cyan as an end group; leading the first to third generation alcohols to react with 4-nitrophthalonitrile respectively, thus synthesizing corresponding phthalocyanine precursor of dendrimer taking the 1-3 generation cyan as the end group; subsequently, leading the phthalocyanine precursor ring of dendrimer taking the 1-3 generation cyan as the end group to synthesize corresponding arylene ether dendritic phthalocyanine taking the first to third generation cyan as the end group and the arylene ether dendritic phthalocyanine taking the first to third generation carboxyl as the end group generated by hydrolysis. The 1-3 generation arylene ether dendritic phthalocyanine complex and the amphiphilic block copolymer are automatically dissolved to form the polymer nano-particle loading the 1-3 generation arylene ether dendritic phthalocyanine complex. The 1-3 generation diaryl ether dendritic phthalocyanine zinc complex and the loaded polymer nano-particle thereof are used as photosencitizers for photodynamic therapy.
Owner:FUJIAN NORMAL UNIV
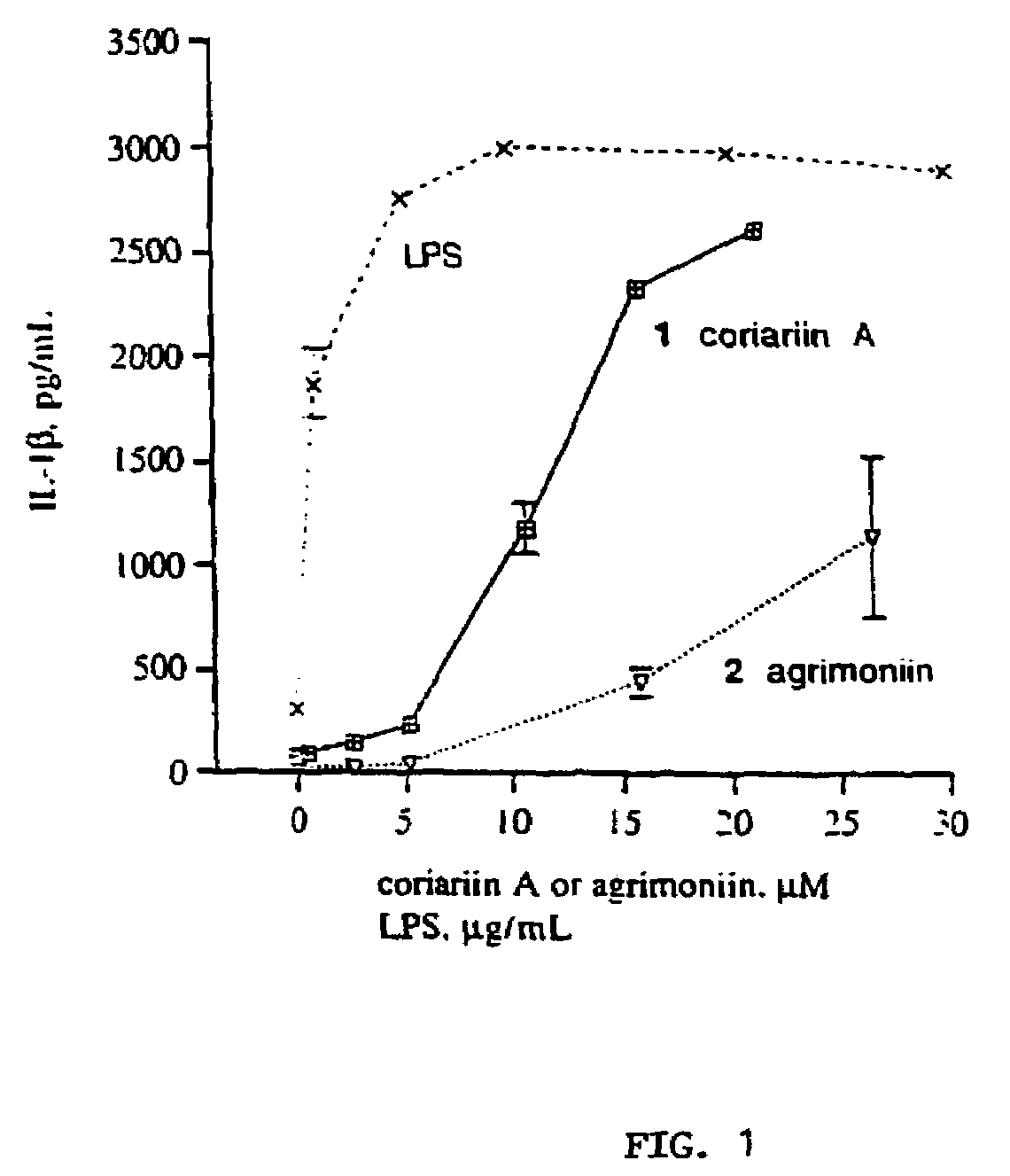
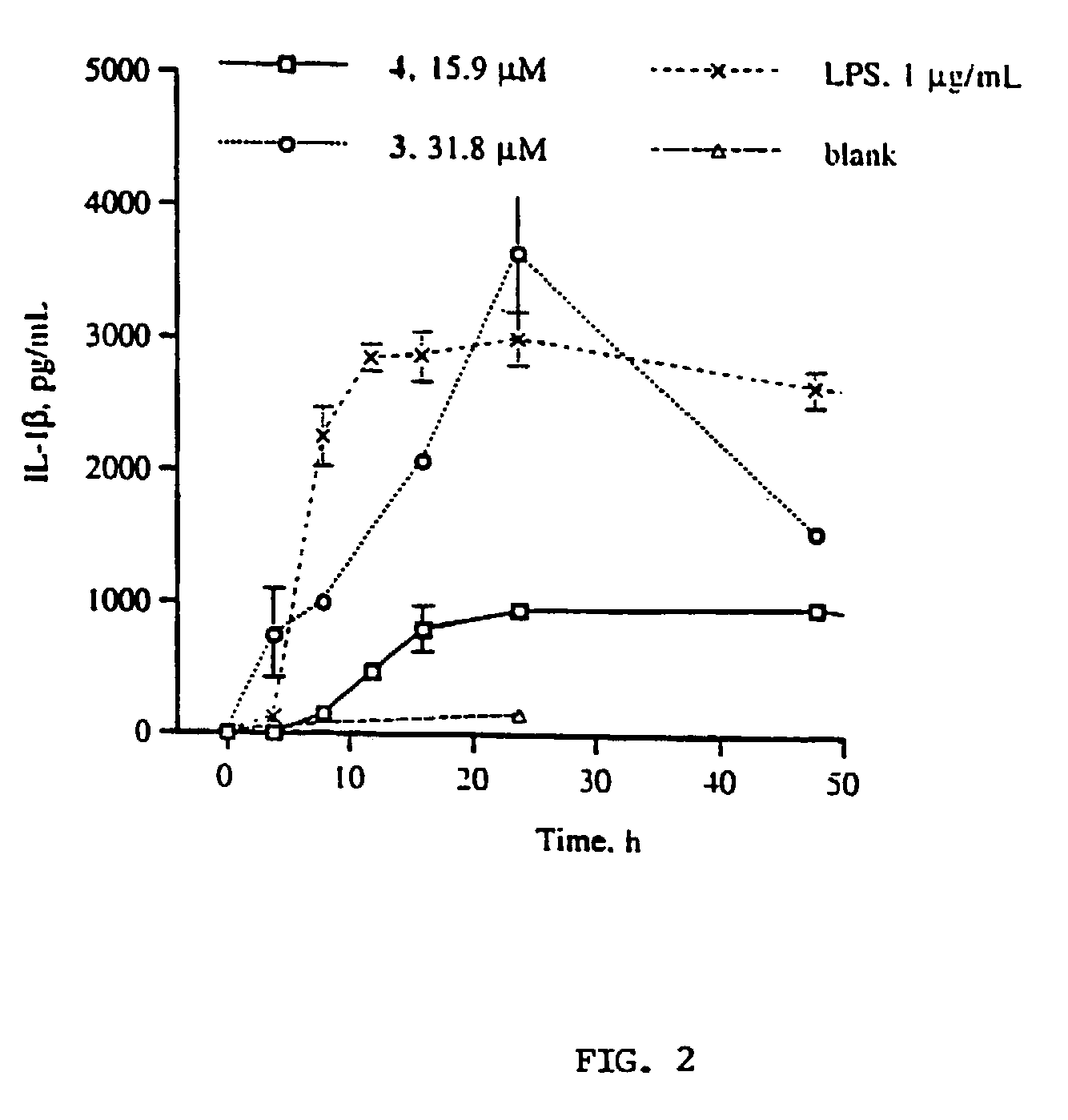
Gallotannins and ellagitannins as regulators of cytokine release
InactiveUS7288273B1Facilitated releaseInduce productionBiocideOrganic active ingredientsFactor iiSecretion
A means and method for increasing or inhibiting the secretion of cytokines using gallotannins and ellagitannins is described. The preferred cytokine release inhibiting compounds are dimeric gallotannins having a linker molecule that misaligns the carbohydrate cores of the compounds. The preferred cytokine release promoting gallotannins and ellagitannins include a diaryl ether linker unit. In comparison to the more structurally complex ellagitannins, the compounds of this invention are structurally simpler, easier to synthesize, and more potent.
Owner:PENN STATE RES FOUND
Diaryl ether condensation reactions
InactiveUS20050054882A1Efficient synthesisDramatic effectCarboxylic acid nitrile preparationOrganic compound preparationLeaving groupDiaryl ether
One aspect of the present invention relates to novel reaction conditions that allow the efficient synthesis of diaryl ethers from arenes bearing a leaving group and arenols under relatively mild conditions. Another aspect of the present invention relates to the dramatic effects of acidic activators on Ullmann-type couplings involving electron-poor and / or relatively insoluble substrates.
Owner:MARCOUX JEAN FRANCOIS
Olefinic polymerization catalyst, preparation method and polymerization method
The invention relates to an olefinic polymerization catalyst, a preparation method and a polymerization method. The olefinic polymerization catalyst comprises three components: A is solid catalyst component, B is organo-aluminum compound, and C is external electron donor and organic silicon compound; wherein, the A component contains titanium, magnesium and chlorine elements as well as internal electron donor which is the combination of 2, 4- diaryl acid pentanediol ester (PBA) and 1, 3-diether (BMF), the combination of the PBA and 1, 4- diaryl ether (BN), or the combination of PBA and aromatic acid ester. The olefinic polymerization catalyst has higher catalytic activity, the activity of the catalyst used for propylene polymerization can be regulated within the range of 40.0-120.0Kg pp / gCat, and isotactic index and molecular weight distribution index of polypropylene are also can be adjusted within the wide range.
Owner:YINGKOU XIANGYANG CATALYST
Method for synthesizing o-amino diaryl ether and o-amino diaryl sulfur ether
InactiveCN102850156AHigh yieldGood compatibilityOrganic compound preparationAmino group formation/introductionPtru catalystSulfur Ethers
The invention discloses a method for synthesizing o-amino diaryl ether and o-amino diaryl sulfur ether. The method comprises the steps of: adding an iron and / or copper catalyst in solvent alcohol or water under the action of alkali, and carrying out open loop coupling reaction on catalytic aryl halide and benzoazole compound to prepare the o-amino diaryl ether or o-amino diaryl sulfur ether, wherein the reaction general formula is shown in the specification. The method for preparing the o-amino diaryl ether and o-amino diaryl sulfur ether by the coupling reaction is low in catalyst price, low in toxicity, direct to react, high in atom economy, wide and stable in substrate source and wide in application scope. Under the optimized reaction conditions, the separation yield of target products reaches up to 96%.
Owner:NANJING NORMAL UNIVERSITY
Catalysts and methods for alcohol dehydration
Provided is a method for preparing a diaryl ether compound through the dehydration of an aromatic alcohol compound in the presence of a dehydration catalyst. The dehydration catalyst comprises an oxide of yttrium.
Owner:THE DOW CHEM CO
Catalysts and methods for alcohol dehydration
InactiveUS9051252B2Catalyst activation/preparationHeat-exchange elementsRare-earth elementAromatic alcohol
Provided is a process for preparing a diaryl ether compound through the dehydration of an aromatic alcohol compound in the presence of a dehydration catalyst. The dehydration catalyst is an oxide of a light rare earth element, wherein the light earth element is lanthanum, praseodymium, neodymium, or mixtures thereof.
Owner:DOW GLOBAL TECH LLC
Method for synthesizing diaryl ether compounds
InactiveCN103342630ASolve the shortcomings of easy oxidation and difficult storageMild reaction conditionsEther preparation by ester reactionsArylDiaryl ether
The invention relates to a preparation method for synthesizing compounds, particularly a method for synthesizing diaryl ether compounds. The invention aims to solve the problem of difficulty in implementing industrialized production due to the defects of complex preparation technique, strict reaction conditions, uncontrollable operation, too many byproducts, and high oxidation tendency and difficulty in storage of the catalyst used in reaction in the existing preparation method for synthesizing diaryl ether compounds. The method comprises the following steps: 1. preparing a diaryl ether compound crude product; and 2. purifying to obtain the diaryl ether compounds. By using the cupric salt which is easy to store and introducing the cheap ligand, the invention saves the cost and is simple to operate; and the invention has the advantages of mild reaction conditions, low temperature and high yield. The invention is used for synthesizing diaryl ether compounds.
Owner:HEILONGJIANG UNIV
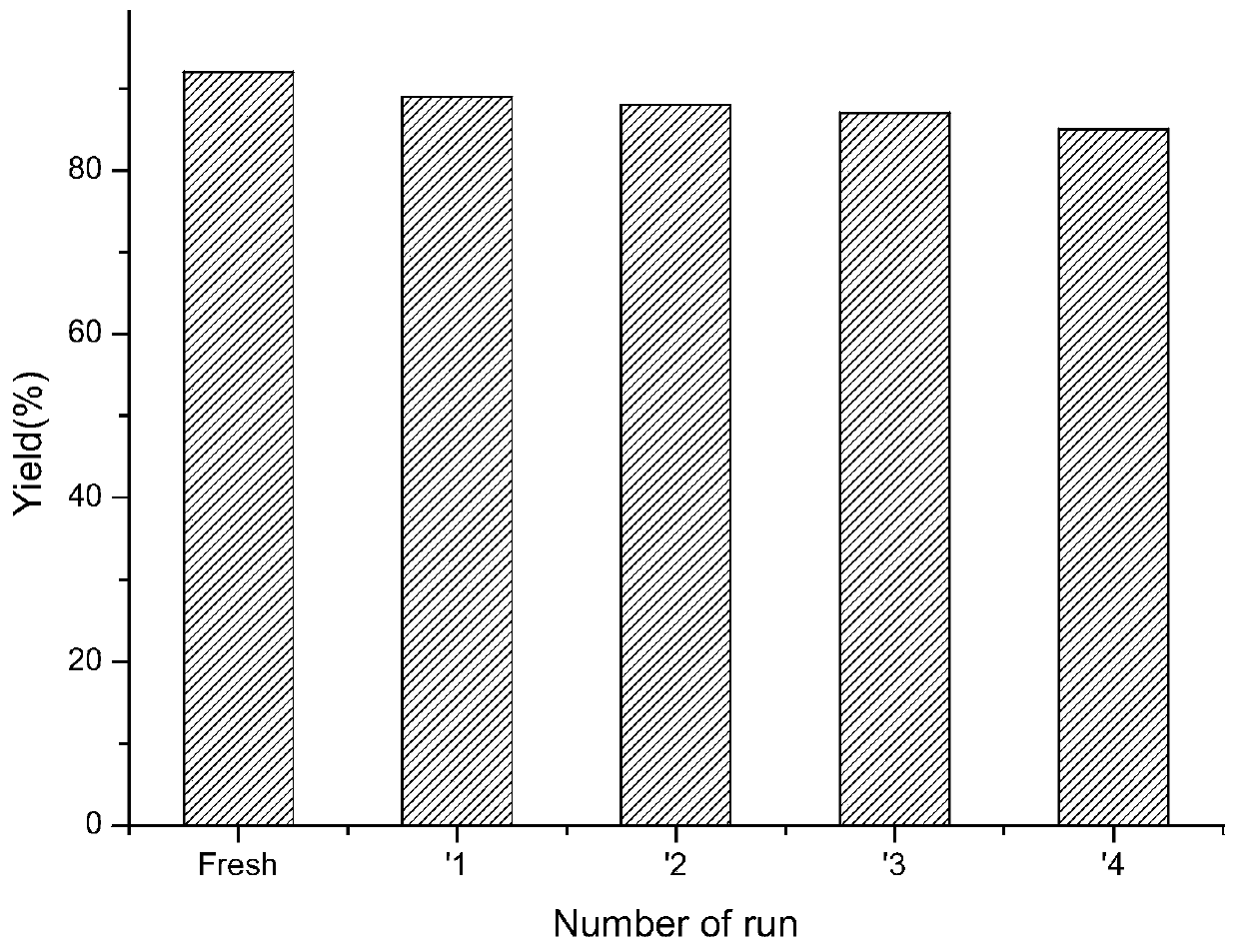
Preparation method of diaryl ether compound
ActiveCN109761762AAchieving zero emissionsReduce manufacturing costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDiaryl etherProcess conditions
The invention relates to a preparation method of a diaryl ether compound. Particularly, under catalysis of chitosan supported cuprous oxide, an arylation reaction used for phenols is achieved, the corresponding diaryl ether compound is obtained, the technological condition is simple, the yield is good, operability is high, and wide functional group durability is achieved.
Owner:YANCHENG TEACHERS UNIV
Catalysts and methods for alcohol dehydration
InactiveUS20150375214A1Organic compound preparationCatalyst activation/preparationArylRare-earth element
Provided is a process for preparing a diaryl ether compound through the dehydration of an aromatic alcohol compound in the presence of a halogenated rare earth element oxide catalyst, providing a reaction vessel having loaded therein a rare earth element oxide; halogenating the rare earth element oxide with a halogen source to form an activated catalyst; and dehydrating an aromatic alcohol compound over the activated catalyst to form the diaryl ether compound, where the halogenating and dehydrating steps occur in the same vessel. The rare earth element oxide is an oxide of a light rare earth element, an oxide of a medium rare earth element, an oxide of a heavy rare earth element, an oxide of yttrium, or a mixture of two or more thereof.
Owner:DOW GLOBAL TECH LLC
Substituted diaryl ester compound, and preparation method and application thereof
InactiveCN104163772ASuppresses the malignant proliferation phenotypeHigh expressionCarboxylic acid nitrile preparationOrganic compound preparationArylHalogen
The invention discloses a substituted diaryl ether compound. The structural formula of the compound is shown in the specification. In the structural formula, Z is -CH- or -N-; W is -CH-or -N-; Y is -O-, -CH2-, -NH- or -NRy-; R1 is one of hydrogen, halogen, NO2, CN, CF3, ORa, CORa, COORa, SO2Ra, SO2NRaRb, NRaRb, NRaCORb, a non-substituted / substituted C1-C4 alkyl group, a non-substituted / substituted aryl group and a non-substituted / substituted heterocyclic group; R2 is hydrogen, a non-substituted / substituted C1-C4 alkyl group, or halogen; and R is one of a non-substituted / substituted C1-C12 alkyl group, a non-substituted / substituted C3-C12 cycloalkyl group, a non-substituted / substituted C2-C12 alkenyl group, a non-substituted / substituted aryl group, substituted alkylamine, and a non-substituted / substituted heterocyclic group. The substituted diaryl ether compound can obviously inhibit the malignant proliferation phenotype of cancer cells, promotes the expression of cell apoptosis factor proteins, and provides a new potential anticancer drug.
Owner:XIAMEN UNIV
Novel preparation method of diaryl ether compound and application thereof
InactiveCN109879779ALow costEasy to operateCarboxylic acid nitrile preparationOrganic compound preparationOrganic synthesisReaction temperature
The invention provides a novel preparation method of a diaryl ether compound. The method concretely comprises the steps of sequentially adding a compound 1, dimethyl sulfoxide, cesium carbonate and acompound 2 into a reactor; performing stirring for 10 to 60 minutes at room temperature; then, putting the materials into an oil bath pot with the reaction temperature of 70 to 120 DEG C; performing illumination by an incandescent light bulb; performing TLC detection on the reaction process; after the reaction is completed, performing filtering, extraction and column chromatography on reaction liquid to obtain a target compound; completing the preparation of the diaryl ether compound. By using the technical scheme, under the visible light induction, no transition metal catalyst and no ligand or photooxidant reducing agent are added; aryl halide and phenol derivatives take photocatalysis C-O crossed coupling reaction. The preparation method has the advantages that the conditions are mild and green; the efficiency is high; the cost is low; the operation is simple and convenient. The prepared compound is an important synthetic intermediate in the fields of biology, medicine and organic synthesis, particularly in an aspect of medicine synthesis.
Owner:CHINA THREE GORGES UNIV
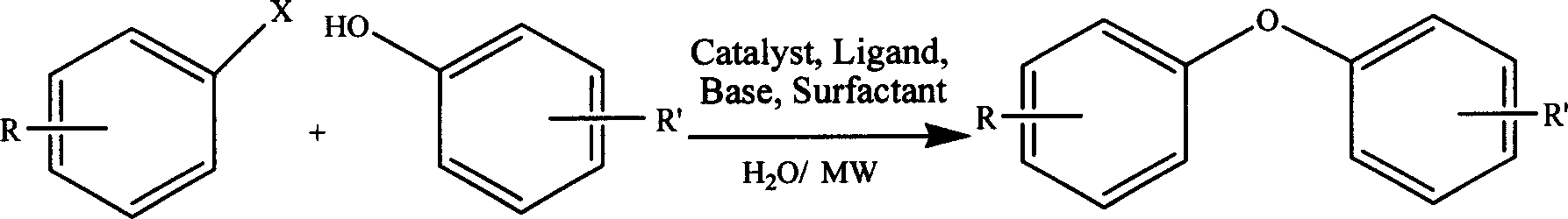
Water heating synthesis of diaryl ether
Synthesis of diaryl ether in water phase is characterized by taking aryl halogenide and phenol as raw material, taking water as solvent, auxiliarily heating by micro-wave, taking carbonate, fluoride, borate and hydroxide of alkali metal or alkaline earth metal as alkali, and C-O coupling reacting under transition metal catalyzing condition or without transition metal catalyzing condition, with ligand or without ligand, with surface activator or without surface activator. It achieves short reacting time, simple operation, easy separation and good reacting environment.
Owner:SUN YAT SEN UNIV
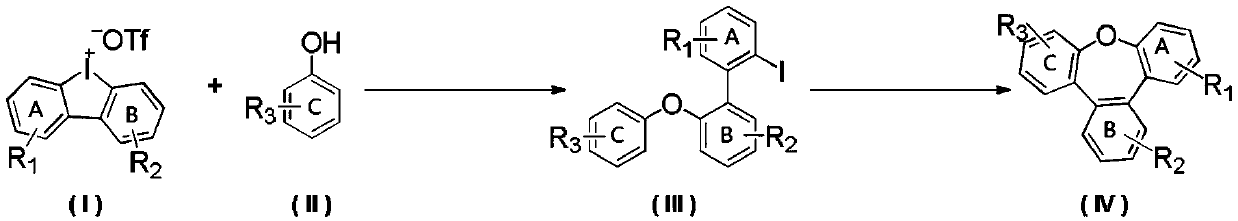
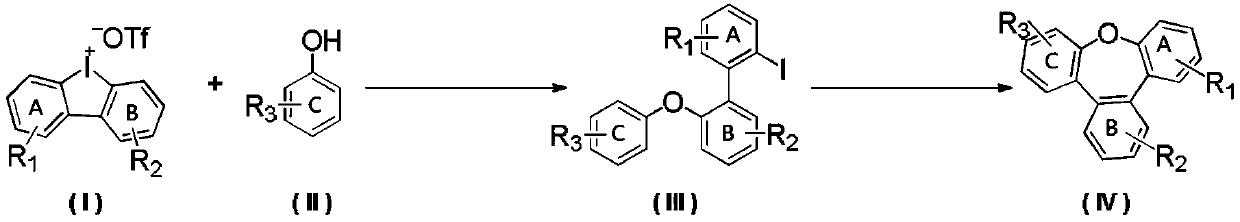
Synthesis method of benzoxepine compound
ActiveCN111116542ABroad biological activitySimple reaction systemOrganic chemistryArylCombinatorial chemistry
The invention provides a synthesis method of a benzoxepine compound. A diarylether compound is efficiently synthesized through a reaction of a diaryl cyclic iodonium salt compound and a substrate containing a hydroxyl group, and the benzoxepine compound is obtained through intramolecular cyclization. The reaction operation is simple, the post-treatment is convenient, and the problems of low efficiency, harsh reaction conditions and the like of a conventional synthesis method are solved; the atom economy of the substrate is fully utilized; and the diarylether compound is efficiently obtained byusing the hydroxyl-containing compound and the diaryl cyclic iodonium salt at a certain temperature under the catalysis of cheap metal copper, intramolecular cyclization is carried out through further reaction, the highest yield of the obtained diarylether compound is 99%, and the highest yield of the benzoxepine compound is 95%.
Owner:ZHEJIANG UNIV OF TECH
Catalysts and methods for alcohol dehydration
Provided is a process for preparing a diaryl ether compound through the dehydration of an aromatic alcohol compound in the presence of a dehydration catalyst. The dehydration catalyst is an oxide of a heavy rare earth element, wherein the heavy rare earth element is terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium, or mixtures thereof.
Owner:DOW GLOBAL TECH LLC
Macrocyclic lactam compound using diaryl ether as skeleton, and preparation method thereof
InactiveCN107540691AEasy to synthesizeImprove biological activityOrganic chemistryArylChemical compound
The invention relates to a macrocyclic lactam compound with a diaryl ether as a skeleton and a preparation method thereof, and mainly solves the technical problem that currently there are few macrocyclic lactam compounds with a diaryl ether as a skeleton. The technical scheme of the present invention: a macrocyclic lactam compound with a diaryl ether as a skeleton is characterized in that it has the following structural formula: R1 and R2 are alkyl, cycloalkyl, aryl or alkyl with heteroatoms and One of the substituents of aryl.
Owner:上海药明康德新药开发有限公司
Effective use method of medicaments and method of preventing expression of side effect
To provide a medicament which efficiently expresses an immunosuppressive agent or an anti-inflammatory agent and reduces expression of side effect.A medicament includes diaryl sulfide or diaryl ether compound having a 2-amino-1,3-propanediol structure having an activity of reducing lymphocytes circulating peripherally, in combination with an immunosuppressive agent and / or an anti-inflammatory agent.
Owner:PRIOTHERA LTD
Diaryl ether derivatives as well as preparation method and application thereof
InactiveCN104876880AImprove bindingStrong antiviral activityOrganic chemistryAntiviralsPharmaceutical drugTherapy HIV
The invention belongs to the technical field of medicine and particularly relates to diary ether derivatives having a general formula I, pharmaceutical salts, hydrates and solvates of the diary ether derivatives, polycrystal or eutectic crystal of the diary ether derivatives, precursors and derivatives having the same biological functions, a preparation method of the diary ether derivatives and application of compositions containing one or more of the compounds in preparation of related medicines for treating Aids. Pharmacological experiment results show that the compounds have remarkable activity of resisting HIV-1 virus, are capable of effectively inhibiting replication of MT-4 cell infected with HIV-1 virus and have relatively low cytotoxicity. The general formula I is shown in the specification.
Owner:FUDAN UNIV
Gallotannins and elligitannins as regulators of cytokine release
InactiveUS20080070850A1Facilitated releaseInduce productionEsterified saccharide compoundsAntibacterial agentsDiaryl etherSecretion
A means and method for increasing or inhibiting the secretion of cytokines using gallotannins and ellagitannins is described. The preferred cytokine release inhibiting compounds are dimeric gallotannins having a linker molecule that misaligns the carbohydrate cores of the compounds. The preferred cytokine release promoting gallotannins and ellagitannins include a diaryl ether linker unit. In comparison to the more structurally complex ellagitannins, the compounds of this invention are structurally simpler, easier to synthesize, and more potent.
Owner:PENN STATE RES FOUND
Catalysts and methods for alcohol dehydration
InactiveCN105263622AOrganic compound preparationCatalyst regeneration/reactivationRare-earth elementAromatic alcohol
Provided is a method for preparing a diaryl ether compound through the dehydration of an aromatic alcohol compound in the presence of a halogenated rare earth element oxide catalyst, wherein the used dehydration catalyst may be regenerated by a halogenation step. The rare earth element oxide is an oxide of a light rare earth element, an oxide of a medium rare earth element, an oxide of a heavy rare earth element, an oxide of yttrium, or a mixtures of two or more thereof.
Owner:DOW GLOBAL TECH LLC
Antidiabetic bicyclic compounds
Diaryl ethers in which one of the aryl groups is a phenyl fused to a cycloalkyl or heterocyclic ring, to which is attached an acetic acid group, are agonists of G-protein coupled receptor 40 (GPR40) and are useful as therapeutic compounds, particularly in the treatment of Type 2 diabetes mellitus, and of conditions that often accompany this disease, including insulin resistance, obesity and lipid disorders.
Owner:MERCK SHARP & DOHME LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com