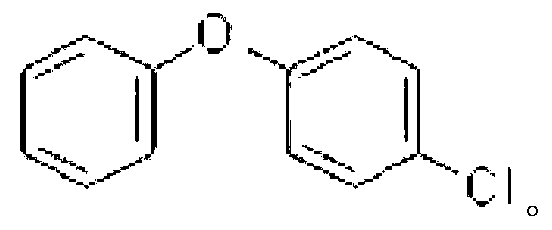
Method for synthesizing diaryl ether compounds
A technology for diaryl ethers and compounds, which is applied in the field of preparation of synthetic compounds, can solve the problems of harsh reaction conditions, many by-products, complicated preparation processes, etc., and achieves the effects of mild reaction conditions, improved selectivity and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0034] Specific embodiment one: a kind of method for synthesizing diaryl ether compound described in this embodiment is specifically carried out according to the following steps:
[0035] 1. Preparation of crude diaryl ether compound: Add picolinic acid, cesium carbonate, sodium ascorbate, polyethylene glycol 400 and dimethyl sulfoxide to copper trifluoroacetate in sequence, and stir and mix for 10-20 minutes, then add phenol Classes and aryl iodides, and then heated from room temperature to 40-60°C under the protection of nitrogen, and reacted at a temperature of 40-60°C for 10h-18h to obtain crude diaryl ether compounds;
[0036] The mol ratio of described phenols and aryl iodide is 1:(1~1.5); The mol ratio of described copper trifluoroacetate and phenols is 1:(5~15); Described picolinic acid and The mol ratio of phenols is 1:(3~8); The mol ratio of described cesium carbonate and phenols is (1.5~2.5):1; The mol ratio of described sodium ascorbate and phenols is 1:(4 ~8); th...
specific Embodiment approach 2
[0043] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the phenols described in step one are phenol, o-cresol, m-cresol, p-cresol, o-methoxyphenol, m-cresol Methoxyphenol or p-methoxyphenol. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0044] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the aryl iodide described in step 1 is iodobenzene, m-chloroiodobenzene, p-chloroiodobenzene or p-bromoiodobenzene. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com