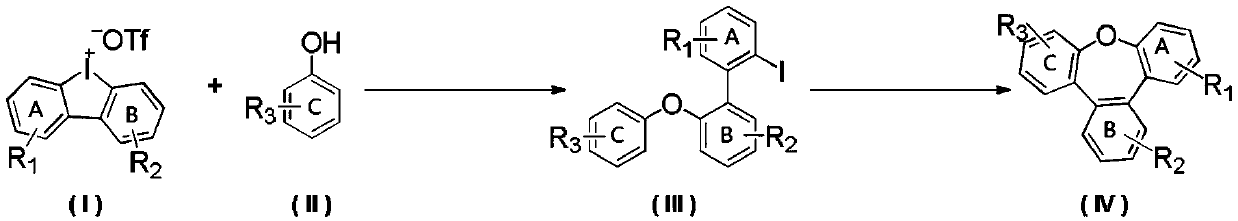
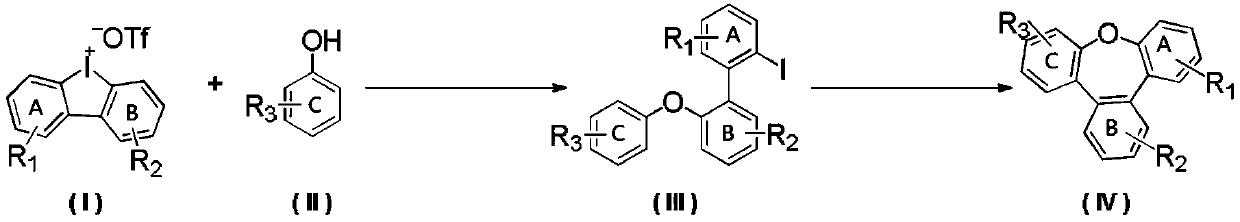
Synthesis method of benzoxepine compound
A technology for benzoxa and salt compounds, which is applied in the field of synthesis of benzoxa compounds, can solve problems such as low efficiency and harsh reaction conditions, and achieve high total yield, simple reaction system, and simple post-treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of 2-iodo-2'-phenoxy-1,1'-biphenyl
[0029] Phenol (45.2mg, 0.48mmol), [1,1'-biphenyl]-cyclic iodonium trifluoromethanesulfonate (171.1mg, 0.4mmol), copper trifluoromethanesulfonate (14.5mg, 0.04mmol ), potassium carbonate (110.5mg, 0.8mmol) were dissolved in 2mL of dichloromethane and placed in a 35mL pressure tube, and the solution was stirred at 100°C for 18h. Concentrate, and go through silica gel column chromatography, eluting with petroleum ether / ethyl acetate = 100:1, and concentrate to obtain 139.5 mg of the product with a yield of 94%. 1 H NMR (500MHz, CDCl 3 )δ7.91(dd, J=8.0,1.1Hz,1H),7.40–7.32(m,2H),7.31(dd,J=7.6,2.1Hz,1H),7.29–7.25(m,3H),7.20 (td,J=7.5,1.2Hz,1H),7.05(tt,J=7.3,1.1Hz,1H),7.03–7.00(m,1H),7.00–6.97(m,3H)ppm; 13C NMR (126MHz, CDCl 3 )δ157.0, 154.1, 143.0, 138.9, 135.8, 131.7, 130.6, 129.5, 129.4, 128.9, 127.7, 123.1, 123.0, 119.1, 118.7, 100.1ppm; HRMS m / z (EI) calcd for C 18 h 13 IO[M] + 372.0011, found: 371.9994. The structural...
Embodiment 2
[0032] Synthesis of Tribenzo[b,d,f]oxepatriene
[0033] 2-iodo-2'-phenoxy-1,1'-biphenyl (74.4mg, 0.2mmol), triphenylphosphine (5.2mg, 0.02mmol), palladium pivalate (3.1mg, 0.01mmol) , potassium acetate (58.8mg, 0.6mmol), and pivalic acid (12.3mg, 0.12mmol) were dissolved in 2mL of N-methylpyrrolidone, and stirred at 130°C for 8h under nitrogen protection. Extraction, concentration, and silica gel column chromatography, eluting with petroleum ether / ethyl acetate = 100:1, and concentration gave 43.2 mg of the product with a yield of 88%. 1 H NMR (500MHz, CDCl 3 )δ7.67(dd, J=5.8,3.4Hz,2H),7.60(dd,J=7.7,1.5Hz,2H),7.52(dd,J=5.8,3.3Hz,2H),7.40–7.33(m ,4H),7.27(ddd,J=7.7,6.6,2.0Hz,2H)ppm; 13 C NMR (126MHz, CDCl 3 )δ160.2, 136.6, 132.9, 129.7, 129.4, 129.3, 128.1, 125.5, 120.9ppm. The structural formula of the product is:
[0034]
Embodiment 3
[0036] Synthesis of 2-iodo-2'-(m-tolyloxy)-1,1'-biphenyl
[0037] According to the method described in Example 1, except that the hydroxyl substrate used was m-cresol (52.0 mg, 0.48 mmol), 150.6 mg of the product was obtained with a yield of 97%. 1 H NMR (500MHz, CDCl 3 )δ7.92(dd,J=7.9,1.1Hz,1H),7.41–7.31(m,3H),7.31–7.27(m,1H),7.23–7.13(m,2H),7.04–6.97(m, 2H), 6.87(ddt, J=7.5, 1.7, 0.9Hz, 1H), 6.83–6.78(m, 2H), 2.31(s, 3H)ppm; 13 C NMR (126MHz, CDCl 3 )δ156.9, 154.2, 143.0, 139.6, 138.9, 135.7, 131.6, 130.6, 129.3, 129.2, 128.8, 127.7, 123.9, 122.8, 119.8, 118.6, 116.1, 100.0, 21.3ppm; HRMS m / z (for C) 19 h 15 IO[M] + 386.0168, found: 386.0158. The structural formula of the product is:
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com