Method for performing cyclized synthesis on multi-substituted indole through elemental iodine promoted enaminone
A technology of enaminone and elemental iodine is applied in the field of cyclization of enaminones promoted by elemental iodine to synthesize polysubstituted indole, and achieves the effects of high product yield, high atom economy, wide biological activity and medicinal value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of 5-methoxy-2-phenyl-3-benzoylindole of the following structural formula
[0017]
[0018] Add 0.1647g (0.5mmol) (Z)-3-(4-methoxyanilino)-1,3-diphenyl-2-en-1-one, 0.1520g (0.6mmol ) elemental iodine, 0.2879g (1.5mmol) cesium acetate, 4mL 1,4-dioxane, stirred and reacted at 80°C for 24 hours, stopped the reaction, cooled down to room temperature naturally, and separated with a silica gel column (the eluent was ethyl acetate ester and petroleum ether volume ratio of 1:5 mixture), to obtain 5-methoxy-2-phenyl-3-benzoyl indole, and its yield was 94%.
[0019] The resulting product was characterized by a Bruker Avance superconducting Fourier digital NMR spectrometer, and the characterization data were: 1 H NMR (600MHz, DMSO) δ12.10 (s, 1H), 7.50 (d, J = 7.2Hz, 2H), 7.42 (d, J = 8.7Hz, 1H), 7.34 (dd, J = 15.5, 7.8Hz ,4H),7.26-7.15(m,5H),6.91(dd,J=8.7,2.4Hz,1H),3.74(s,3H); 13 C NMR (151MHz, DMSO) δ192.03, 155.12, 144.62, 139.97, 131.70, 131.05, 130.84, 129.4...
Embodiment 2
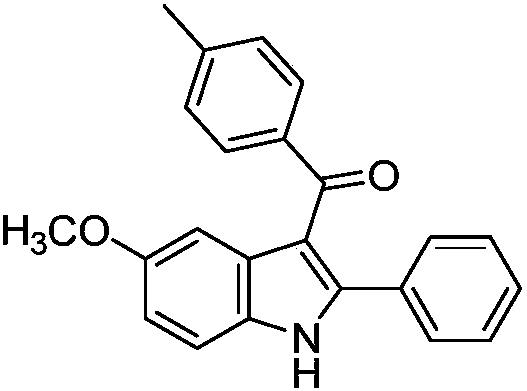
[0027] Preparation of 5-methoxy-2-phenyl-3-(4-methylbenzoyl) indole of the following structural formula
[0028]
[0029] In Example 1, the (Z)-3-(4-methoxyanilino)-1,3-diphenyl-2-en-1-one used was equimolar with (Z)-3-( 4-methoxyanilino)-3-phenyl-1-(4-methylphenyl)-2-en-1-one is replaced, and other steps are the same as in Example 1 to obtain 5-methoxy-2 -Phenyl-3-(4-methylbenzoyl)indole in 98% yield.
[0030] The resulting product was characterized by a Bruker Avance superconducting Fourier digital NMR spectrometer, and the characterization data were: 1 H NMR (600MHz, CDCl 3 )δ8.98(s,1H),7.43(d,J=8.0Hz,2H),7.37(d,J=2.1Hz,1H),7.23-7.14(m,3H),7.06(t,J=7.3 Hz,1H),7.01(t,J=7.3Hz,2H),6.84(d,J=7.9Hz,2H),6.80(dd,J=8.8,2.4Hz,1H),3.69(s,3H), 2.17(s,3H); 13 C NMR (151 MHz, CDCl 3 )δ 193.41, 155.85, 144.08, 142.09, 136.98, 131.86, 130.65, 129.87, 129.49, 129.15, 128.41, 128.23, 113.96, 113.61, 112.01, 102.92, 55.72, 21.48
Embodiment 3
[0032] Preparation of 5-methoxy-2-phenyl-3-(4-nitrobenzoyl) indole of the following structural formula
[0033]
[0034] In Example 1, the (Z)-3-(4-methoxyanilino)-1,3-diphenyl-2-en-1-one used was equimolar with (Z)-3-( 4-methoxyanilino)-3-phenyl-1-(4-nitrophenyl)-2-en-1-one is replaced, and other steps are the same as in Example 1 to obtain 5-methoxy-2 -Phenyl-3-(4-nitrobenzoyl)indole in 97% yield.
[0035] The resulting product was characterized by a Bruker Avance superconducting Fourier digital NMR spectrometer, and the characterization data were: 1 H NMR (600MHz, DMSO) δ12.28(s, 1H), 7.93(d, J=8.7Hz, 2H), 7.61(d, J=8.7Hz, 2H), 7.57(d, J=2.4Hz, 1H ), 7.43(d, J=8.8Hz, 1H), 7.32-7.26(m, 2H), 7.22(t, J=7.3Hz, 1H), 7.17(t, J=7.3Hz, 2H), 6.94(dd ,J=8.8,2.5Hz,1H),3.79(s,3H); 13 C NMR(151MHz,DMSO)δ190.08,155.65,147.99,146.42,146.00,131.30,130.81,129.94,129.91,128.71,128.62,127.86,122.61,113.18,115.77,111.28,
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com