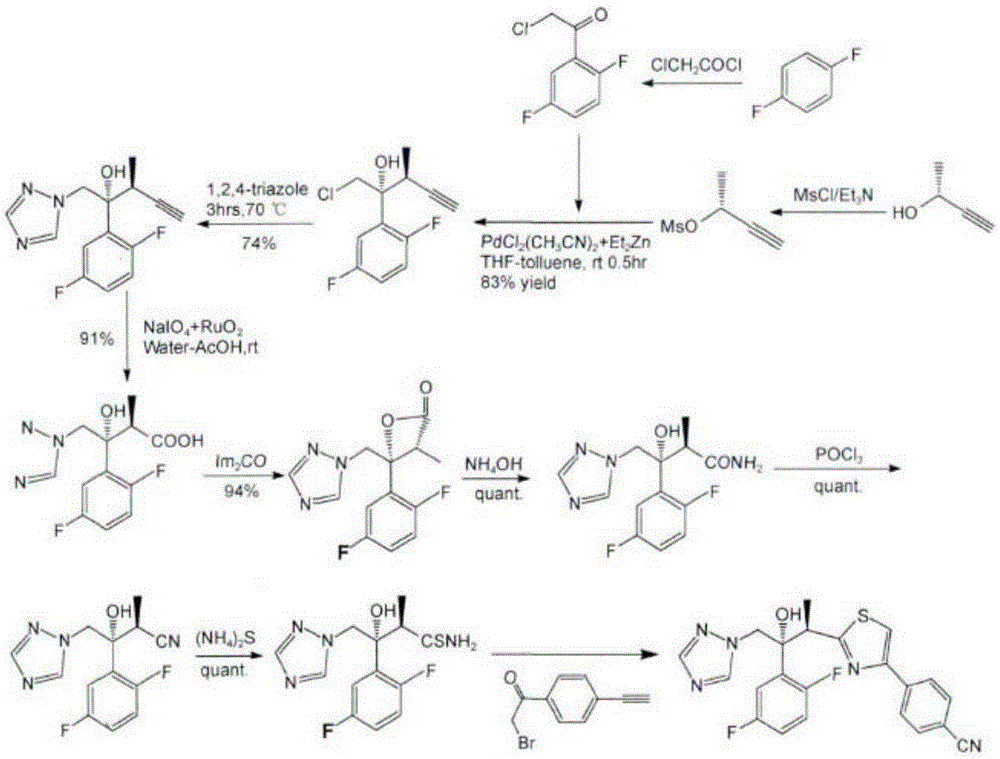
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38results about How to "High split yield" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
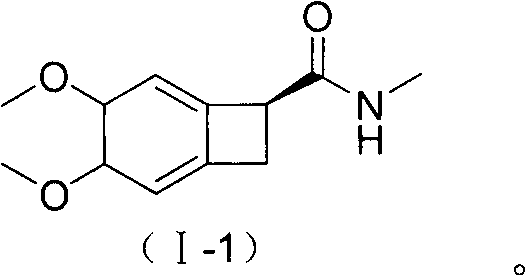
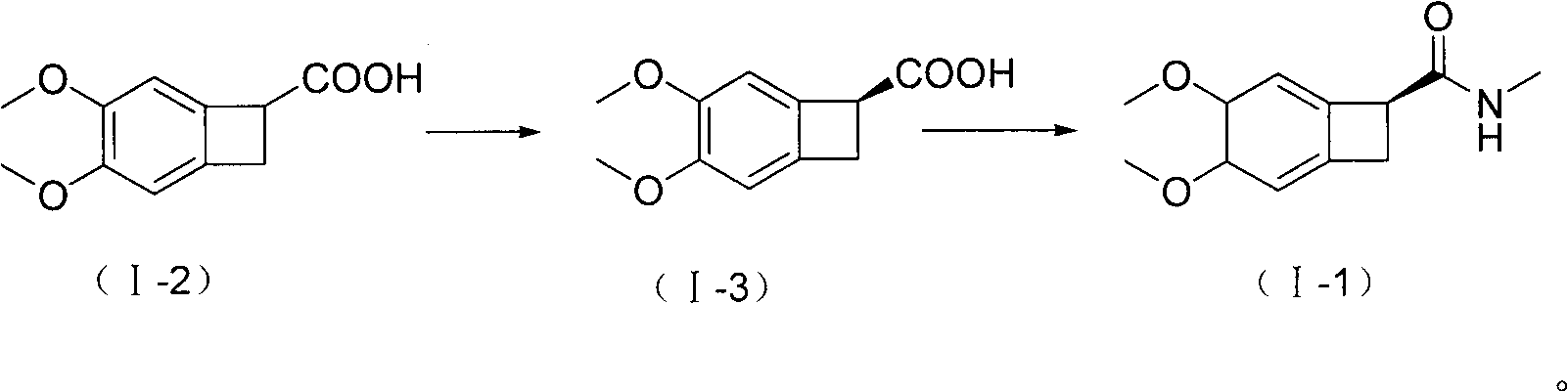
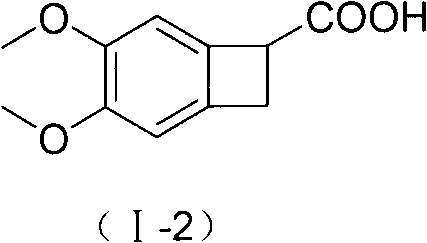
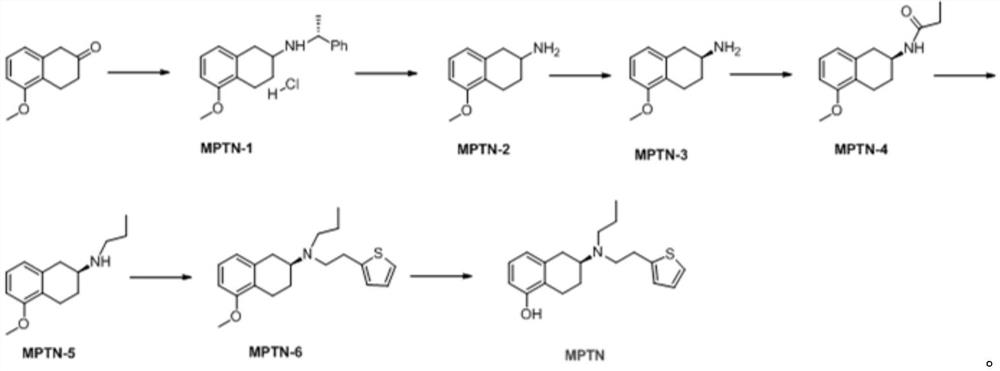
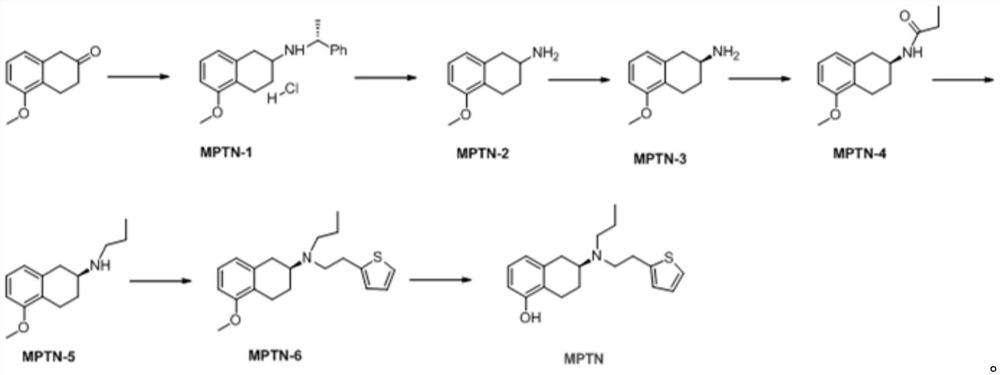
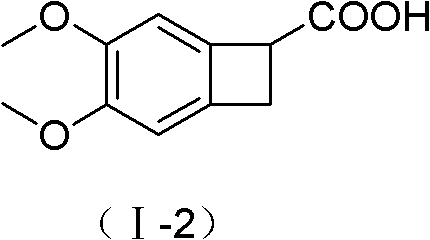
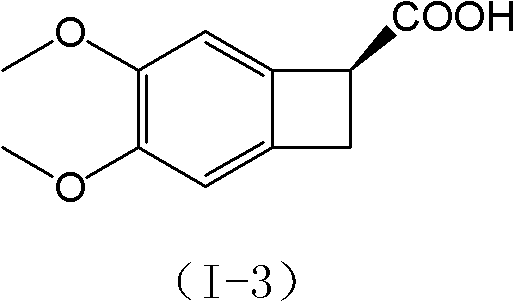
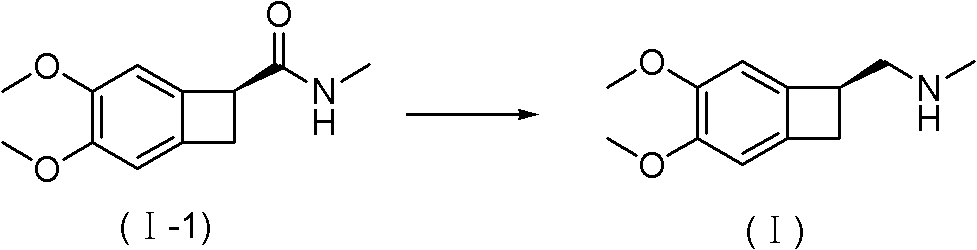
New benzocyclobutane, preparation method thereof and application thereof
InactiveCN101671265AEasy to getMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationIVABRADINE HYDROCHLORIDECombinatorial chemistry
The invention relates to new benzocyclobutane, a preparation method thereof and application thereof. The invention provides a new preparation method for (1S)-4, 5-dimethoxy-1-(methyl-amino-methyl)-benzocyclobutane serving as a key intermediate of ivabradine hydrochloride and addition salt thereof, and meanwhile provides a new benzocyclobutane compound which is an intermediate for preparing the (1S)-4, 5-dimethoxy-1-(methyl-amino-methyl)-benzocyclobutane. In addition, the invention also provides a method for preparing the new benzocyclobutane compound, and meanwhile provides a method for splitting an intermediate product during preparing the new benzocyclobutane compound.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
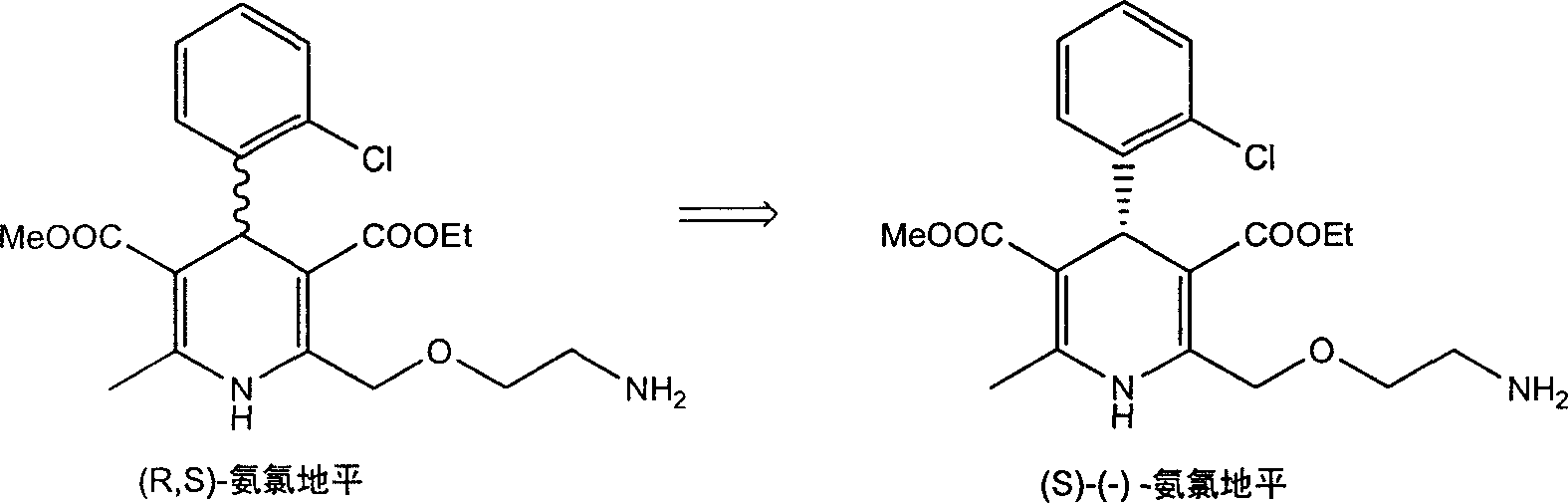
Method for splitting Amlodipine
This invention discloses a method for resolving amlodipine. The method uses tartaric acid as the chiral reagent, and N-methylpyrrolidone as the chiral aid. The method has such advantages as high resolving rate, high product optic purity and simple process, and is suitable for mass production.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA +1
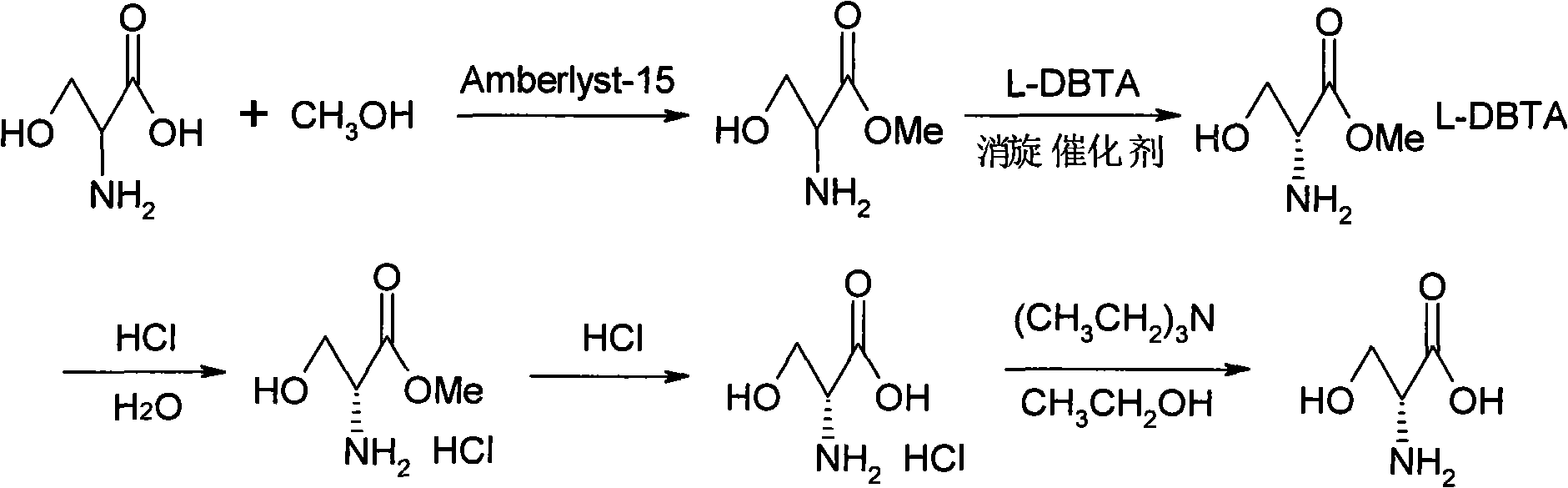
Method for preparing D-serine by kinetic resolution
ActiveCN101735085AHigh split yieldLower operating costsOrganic compound preparationAmino-carboxyl compound preparationMethanolAmberlyst-15
The invention discloses a method for preparing D-serine by kinetic resolution. The method takes DL-serine as raw material and is implemented by esterifying DL-serine with methanol under catalysis of Amberlyst-15 ion exchange resin to obtain DL-serinemethylester, carrying out dynamic kinetic resolution on DL-serinemethylester and resolving agent L-DBTA under action of racemization catalyst to obtain dibasic DL-serinemethylester.L-DBTA, dissociating with hydrochloric acid, and hydrolyzing to obtain D-serine, the target product of the invention. Compared with the prior art, by using the racemization catalyst in the invention, the dynamic continuous conversion of dibasic L-serinemethylester.L-DBTA into dibasic DL-serinemethylester.L-DBTA can be realized with the theoretic conversion rate being approximate to 100%, thus greatly enhancing yield of resolution, reducing operation cost, stabilizing product quality and being suitable for industrialized production.
Owner:SHANGHAI SHISI CHEM PROD
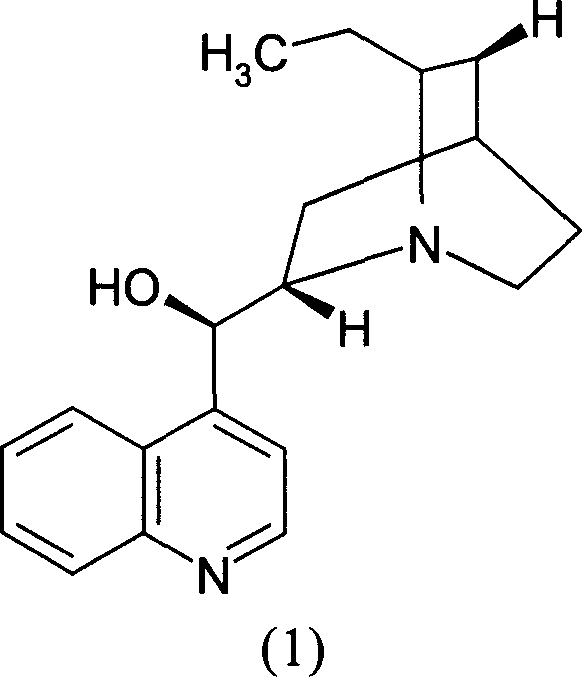
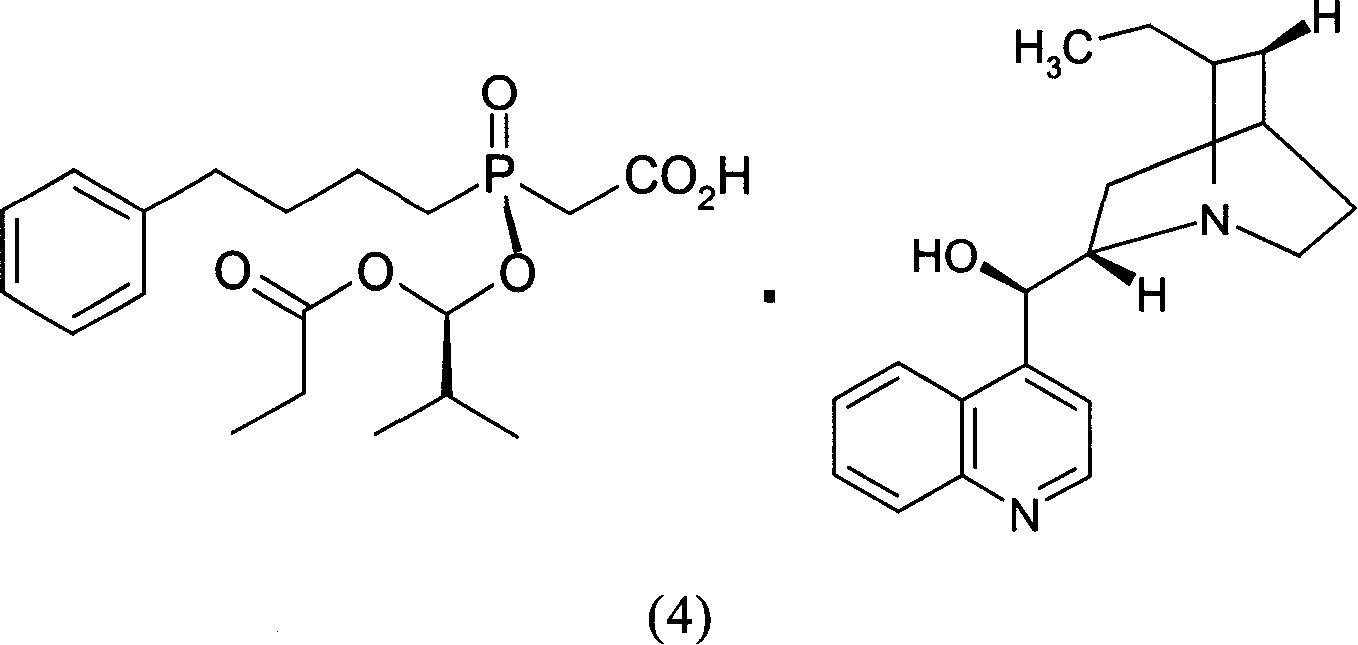
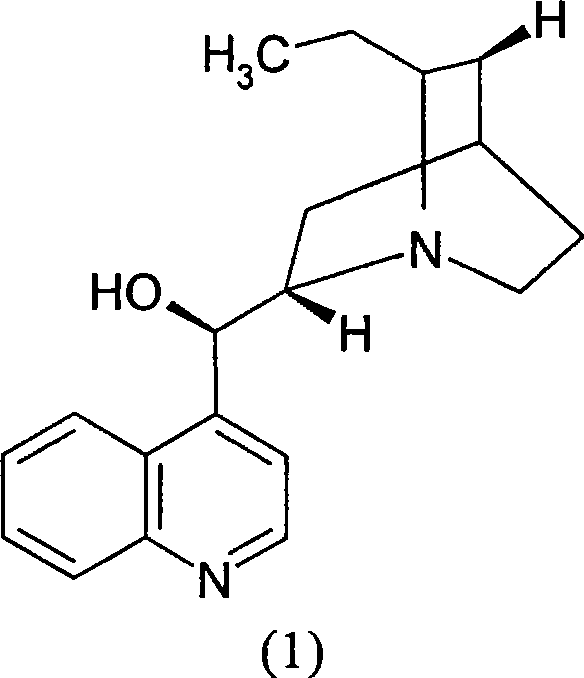
Optical active substitution oxyphosphonate salt acetate and its use
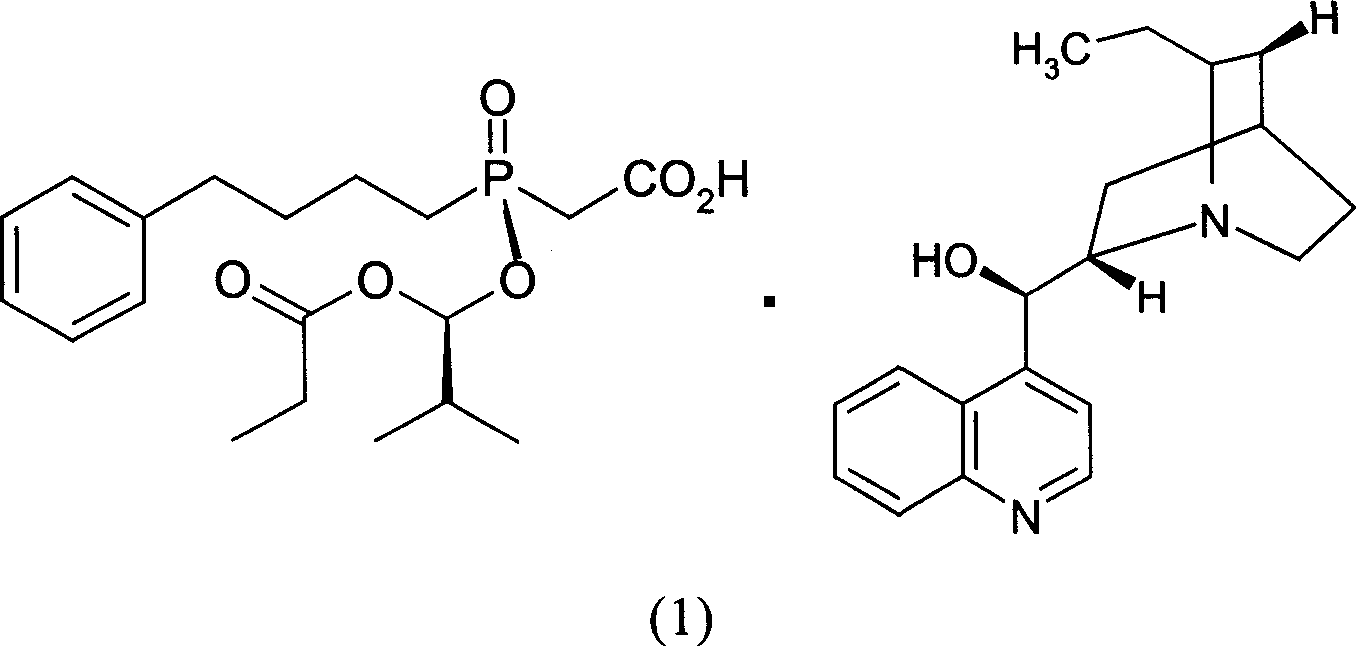
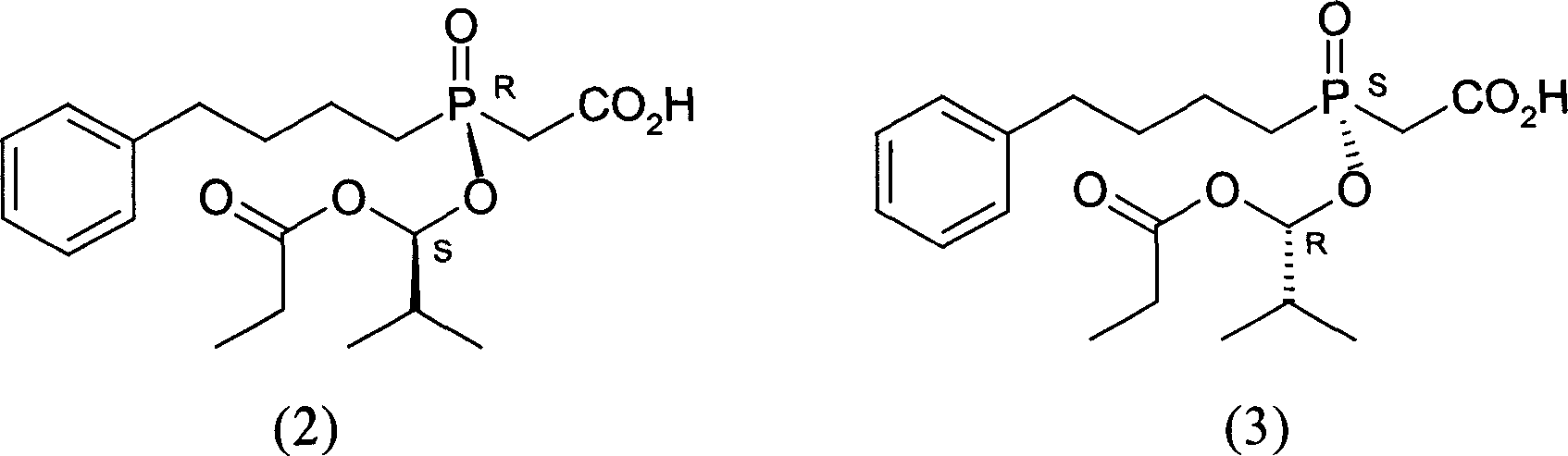
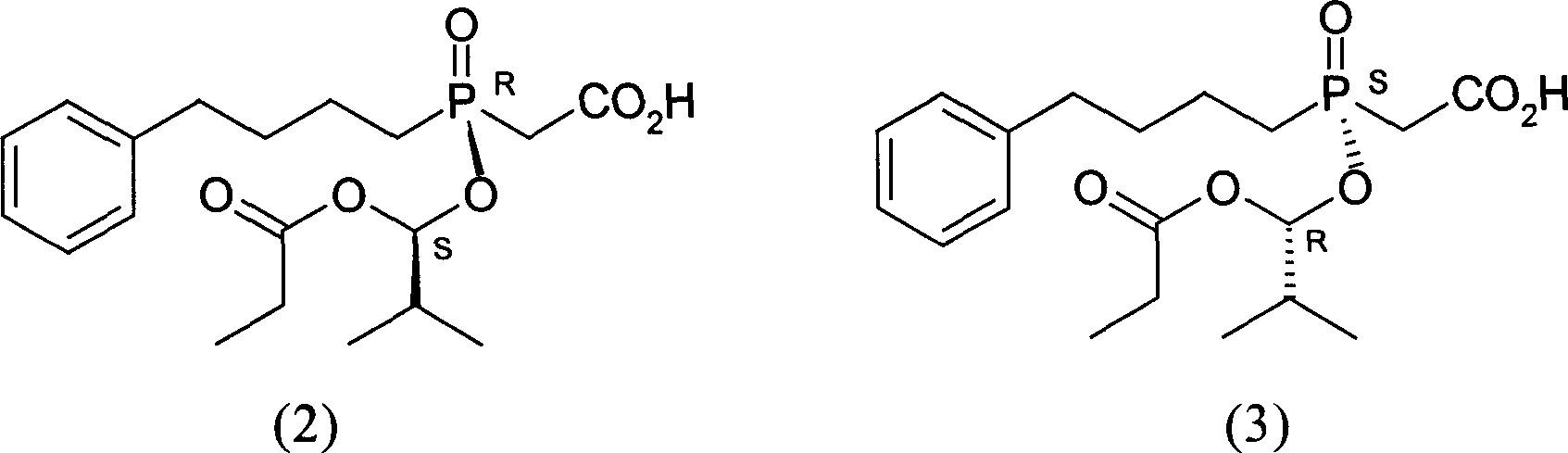
InactiveCN1955185AHigh optical purityReduce consumptionGroup 5/15 element organic compoundsAcetic acidBenzene
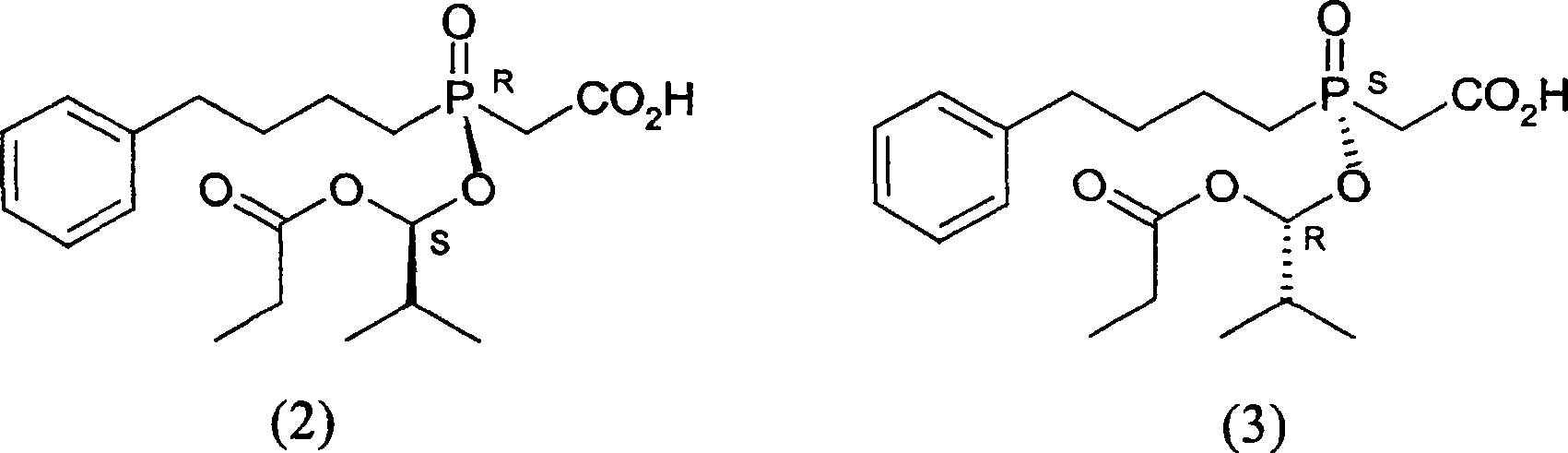
This invention provides a salt that is produced by [(R)-[(1S)-2-methyl-1- (1-keto-propoxy)propoxy](4-benzene butyl) oxygen phosphino]acetic acid and 10,11-di-H Cinchonidine, its structure is shown by formula(1). This invention also provides preparation of using this salt to optically split racemoid which consists of [(R)-[(1S)-2-methyl-1-(1-keto-propoxy)propoxy](4-benzene butyl) oxygen phosphino] acetic acid and [(S)-[(1R)-2-methyl-1-(1-keto-propoxy) propoxy](4- benzene butyl) oxygen phosphino] acetic acid. Compared with present resolution preparation, the yield and optical purity of resolution is obviously raised, it needs not recryst many times to depurate, and dosis of resolution agent is reduced.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
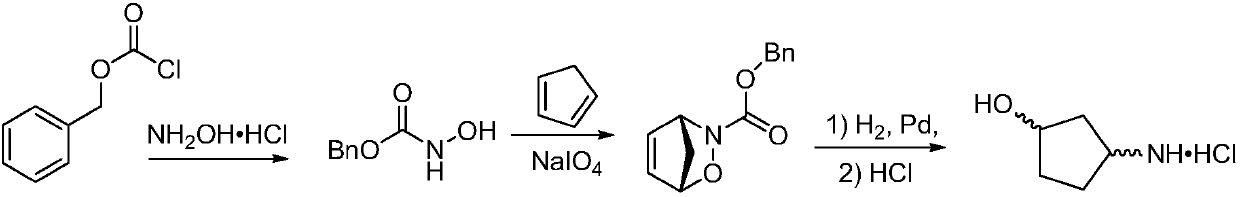
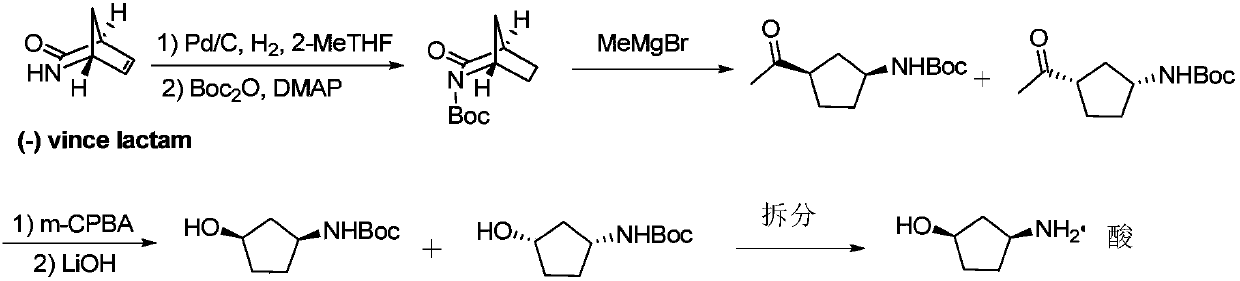
Method for preparing single-configuration 3-aminocyclopentanol through chiral resolution
InactiveCN109988073AMild reaction conditionsEasy to operateOrganic compound preparationOrganic chemistry methodsReagentStereochemistry
The invention relates to a method for preparing a single-configuration 3- aminocyclopentanol through chiral resolution. Specifically, the invention discloses a chiral splitting method of 3-aminocyclopentanol. The preparation method is simple to operate, a used resolving reagent is low in price, the reaction condition is mild, the resolving yield can reach 40%, the optical purity is not lower than99.5%, and the preparation method is suitable for industrial production.
Owner:SHANGHAI DESANO PHARMA INVESTMENT +2
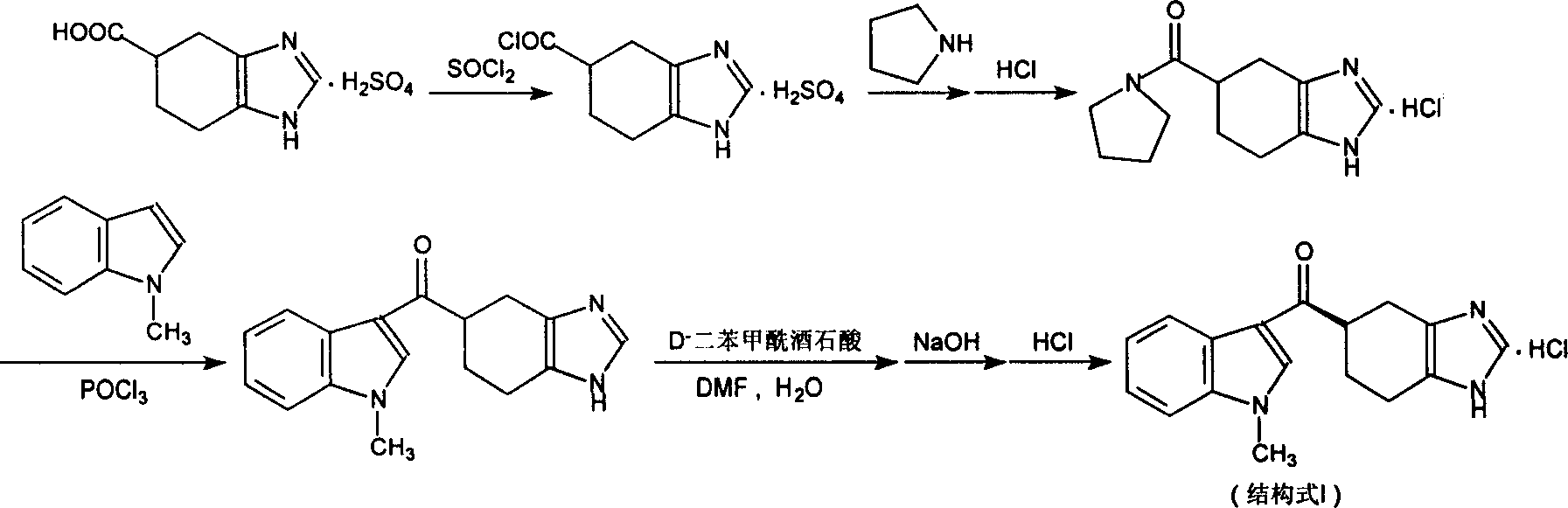
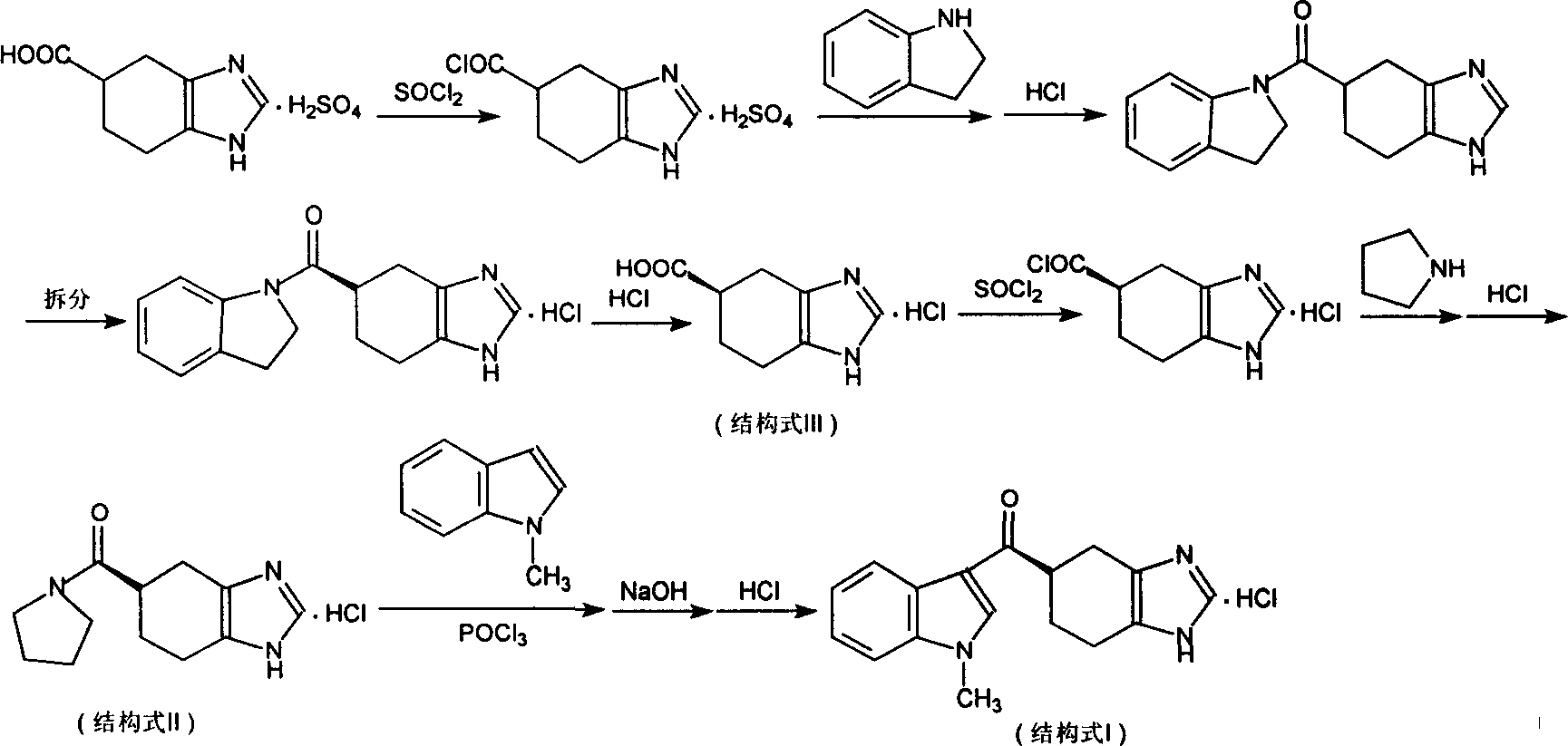
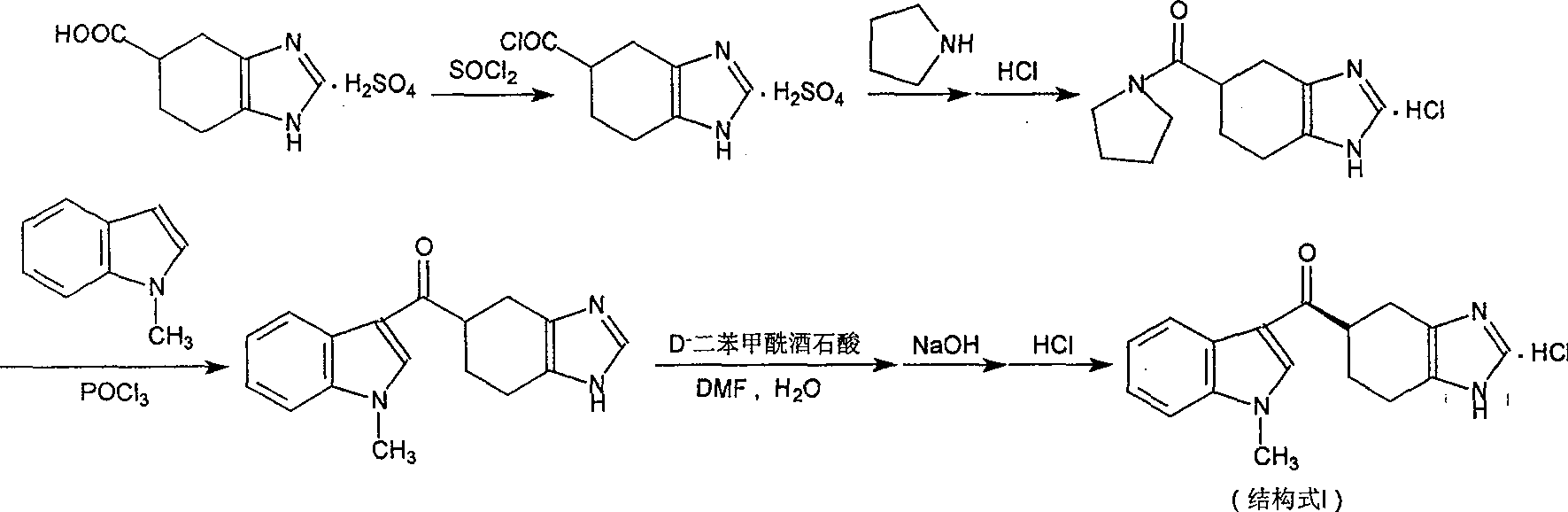
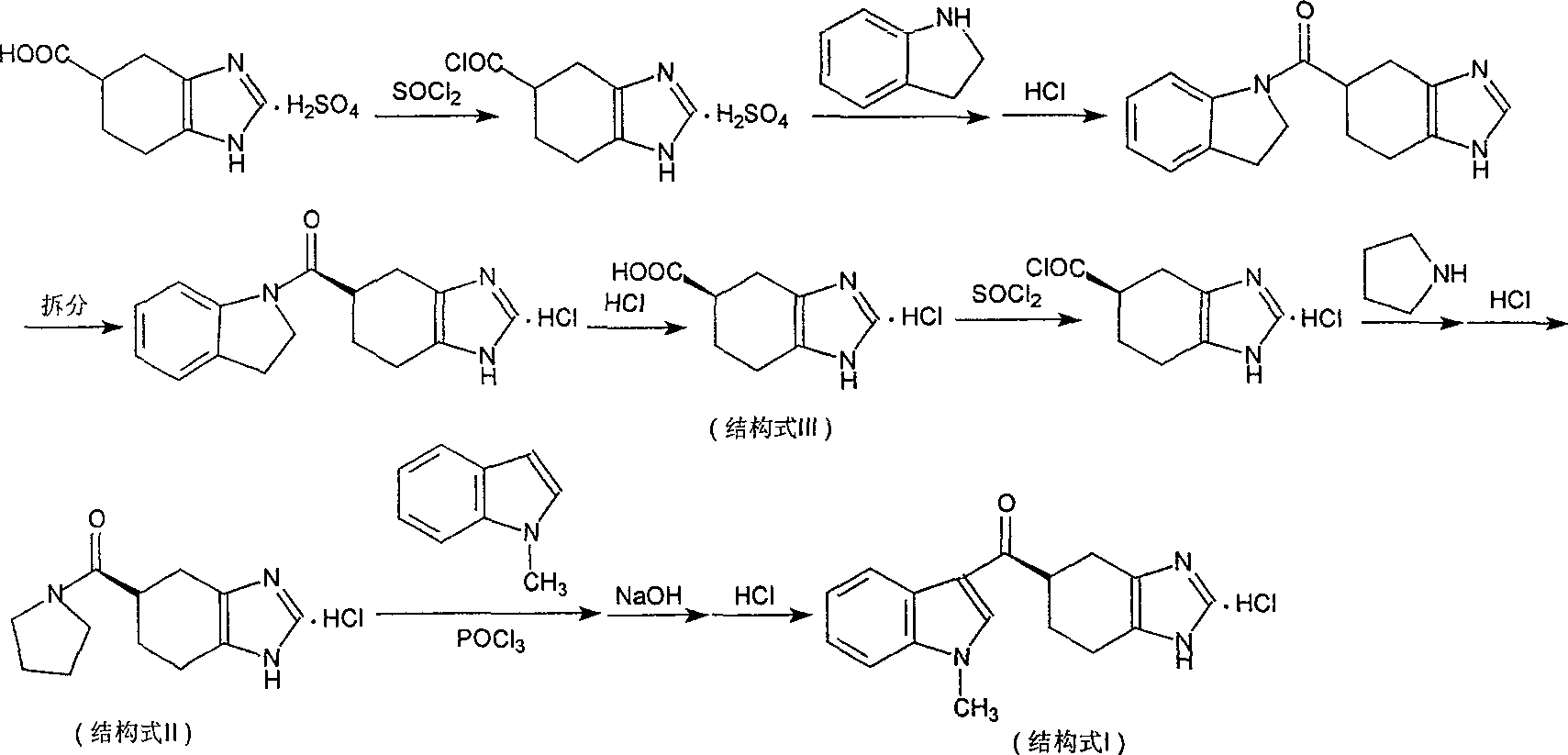
Novel preparation method of ramosetron hydrochloride
The invention describes the preparation method for R-5-[(1-methyl -1H-indole -3-group) carbonyl ]- 4ú¼5ú¼6ú¼7- tetrahydro -1H-benzimidazole (named Ramosetron) as 5-HT3 acceptor antagonist, and its hydrochlorate. Wherein, it uses first-resolution latter-condensation technique, reduces greatly the consumption of N- methylindole, increases total yield, and fits to industrial production.
Owner:TIANJIN ZHONGRUI PHARMA
Aminobutanol resolution method
InactiveCN107245038AHigh specific rotationHigh split yieldOrganic compound preparationOptically-active compound separationSpecific rotationTartrate
The invention relates to an aminobutanol resolution method, which comprises: 1) carrying out a reaction: dissolving L-tartaric acid in anhydrous methanol, heating to a temperature of 65-55 DEG C, adding DL-2-aminobutanol to the L-tartaric acid methanol solution in a dropwise manner, carrying out a reaction, and controlling the pH value of the reaction system at 5-7; 2) precipitating D-2-aminobutanol-tartrate: cooling the mixing liquid obtained in the step 1) to a temperature of 35-25 DEG C to precipitate the D-2-aminobutanol-tartrate; 3) precipitating L-2-aminobutanol-tartrate: evaporating the filtrate in the step 2) to remove the methanol, adding water, controlling the temperature at 55-45 DEG C to obtain a clarified liquid, and cooling to a temperature of 15-5 DEG C so as to precipitate the L-2-aminobutanol-tartrate; and 4) preparing D-2-aminobutanol and L-2-aminobutanol: respectively adding an alkali liquid to the D-2-aminobutanol-tartrate precipitated in the step 2) and the L-2-aminobutanol-tartrate precipitated in the step 3), carrying out alkali precipitation, and respectively rectifying the filtrates so as to obtain the D-2-aminobutanol having the specific rotation of more than or equal to + / -10 and the L-2-aminobutanol having the specific rotation of more than or equal to + / -10.
Owner:嘉兴润博化工科技有限公司 +1
Method for preparing L-2-aminopropanol by means of splitting DL-2-aminopropanol
InactiveCN106810458AIncrease profitHigh split yieldOrganic compound preparationOrganic chemistry methodsDistillationFiltration
The invention relates to a method for preparing L-2-aminopropanol by means of splitting DL-2-aminopropanol. The method includes steps of 1), dissolving L-tartaric acid in water to obtain L-tartaric acid aqueous solution; 2), dissolving the DL-2-aminopropanol in alcohol solvents at the room temperature, then stirring the DL-2-aminopropanol and the alcohol solvents under cooling conditions, simultaneously completely dropwise adding the L-tartaric acid aqueous solution into the DL-2-aminopropanol to obtain mixed solution, then cooling the mixed solution until the temperature of the mixed solution reaches 0-5 DEG C, and preserving heat to obtain cooled solution; 3), adding a small quantity of crystal seeds into the cooled solution, allowing the cooled solution to stand still at the temperatures ranging from -15 DEG C to +25 DEG C, crystallizing the cooled solution at the temperatures ranging from -15 DEG C to +25 DEG C for 16-24 hours and separating out L-tartaric acid-L-2-aminopropanol acid salt crystals; 4), dissolving the L-tartaric acid-L-2-aminopropanol acid salt crystals by the aid of alcohol solvents, adding inorganic bases into the L-tartaric acid-L-2-aminopropanol acid salt crystals in batches, stirring the inorganic bases and the L-tartaric acid-L-2-aminopropanol acid salt crystals until amino alcohol is completely free, carrying out suction filtration and carrying out reduced-pressure distillation on filter liquid to obtain the L-2-aminopropanol. The method has the advantages that processes are easy to implement, raw materials are high in utilization rate, and accordingly the method is suitable for large-scale production.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Preparation method of levo cloperastine fendizoate
The invention discloses a preparation method of levo cloperastine fendizoate, which comprises the following steps: carrying out nucleophilic substitution reaction on 4-chlorobenzhydrol and 2-chloroethanol in a benzene organic solvent, so that an intermediate product is obtained; reacting the intermediate product with piperidine, so that racemic cloperastine is obtained; resolving the racemic cloperastine by using a resolving agent in a fatty alcohol solvent, so that levo cloperastine is obtained; and carrying out salt forming reaction on the levo cloperastine and a fendizoic acid, so that levo cloperastine fendizoate is obtained, wherein the resolving agent is an R-substituted dibenzoyl-L-tartaric acid, and R refers to alkyl, alkoxy, -Cl, -F, -Br or -H. In the method provided by the invention, in a fatty alcohol solvent, an R-substituted dibenzoyl-L-tartaric acid is adopted as a resolving agent for carrying out resolving on racemic cloperastine, and the resolving yield is high, so that the obtained levo cloperastine fendizoate has high optical purity, and has a high product yield.
Owner:CHONGQING HENG AN CHEM CO LTD
Preparation method of rotigotine
ActiveCN113234057AReduce lossHigh split yieldOrganic compound preparationOrganic chemistry methodsTetraloneMandelic acid
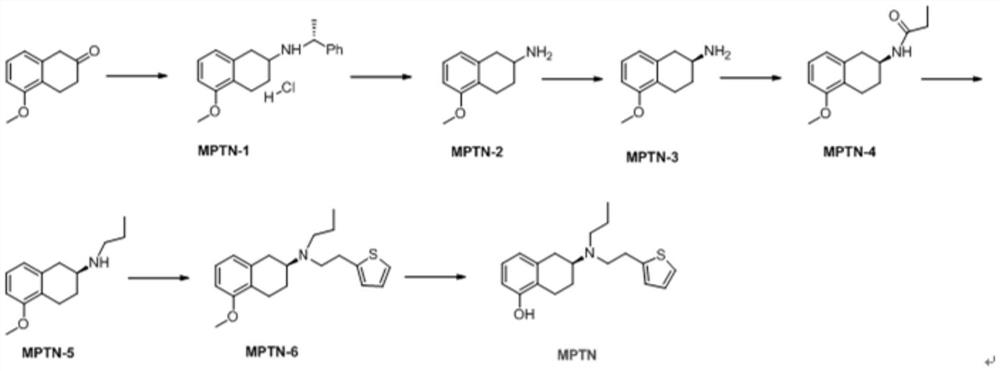
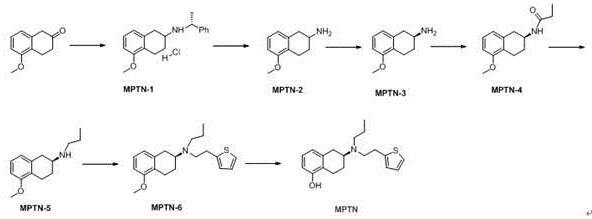
The invention relates to the technical field of medicine preparation, and discloses a preparation method of rotigotine, which comprises the following steps: by taking 5-methoxy-2-tetralone as an initial raw material, reacting with R-alpha-methylbenzylamine, performing debenzylation reduction and S-mandelic acid chiral resolution, then reacting with a propionyl chloride reagent to generate an amide compound, and then reducing by a sodium borohydride reagent to obtain the rotigotine; and finally, reacting with 2-(thiophene-2-yl) 2-nitric acid benzene sulfonic acid ethyl ester to obtain the rotigotine. The preparation process route is as follows: the rotigotine is mild in preparation condition, simple and convenient to operate, relatively high in yield of key intermediates, high in optical purity and easy for industrial large-scale production, and has a very good application prospect.
Owner:CHENGDU TECH UNIV +1
Optical separation method substituting oxyphosphonate acetate
InactiveCN1955176AHigh optical purityReduce consumptionGroup 5/15 element organic compoundsBenzeneAcetic acid
This invention provides a optical resolution preparation that substitutes oxygen phosphino-acetic acid, concretely relating to optical resolution preparation of racemoid which consists of [(R)-[(1S)-2-methyl-1-(1-keto-propoxy) propoxy] ( 4-benzene butyl)oxygen phosphino]acetic acid and [(S)-[(1R)-2-methyl-1- (1-keto-propoxy)propoxy](4- benzene butyl)oxygen phosphino]acetic acid. The preparation of this invention uses 10,11-di-H Cinchonidine shown in following formula(1) as resolution agent, compared with present resolution preparation, the yield and optical purity of resolution is obviously raised, it need not recryst many times to depurate, and dosis of resolution agent is reduced.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Novel preparation method of ramosetron hydrochloride
The invention describes the preparation method for R-5-[(1-methyl -1H-indole -3-group) carbonyl ]- 4,5,6,7- tetrahydro -1H-benzimidazole (named Ramosetron) as 5-HT3 acceptor antagonist, and its hydrochlorate. Wherein, it uses first-resolution latter-condensation technique, reduces greatly the consumption of N- methylindole, increases total yield, and fits to industrial production.
Owner:TIANJIN ZHONGRUI PHARMA
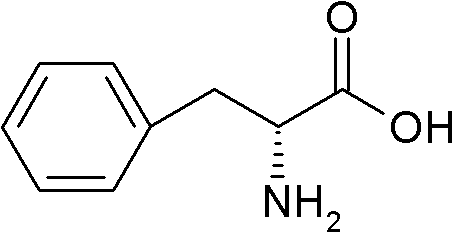
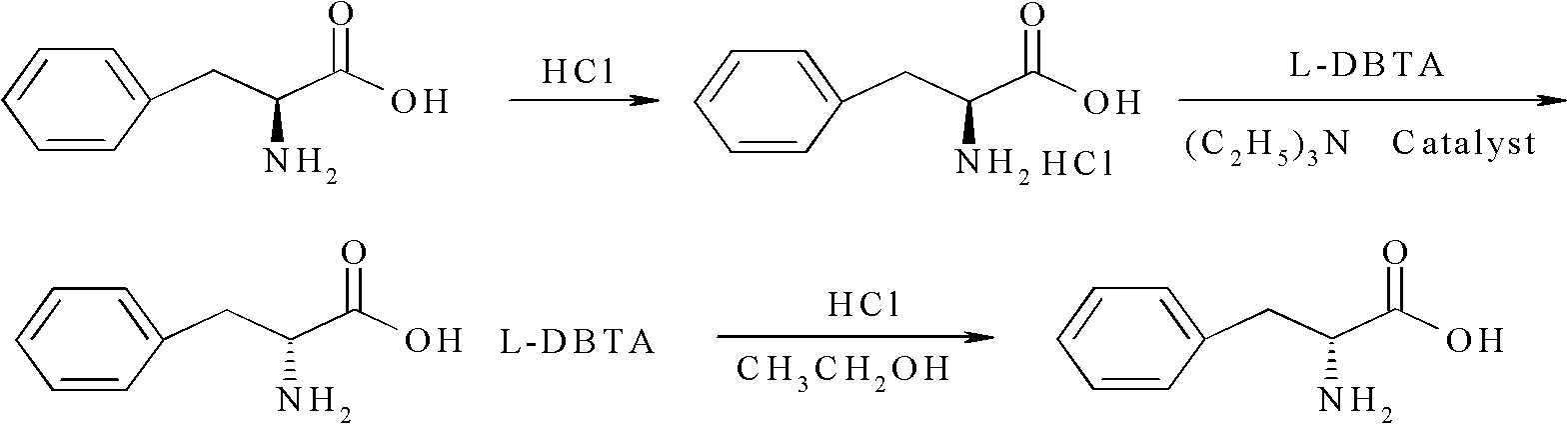
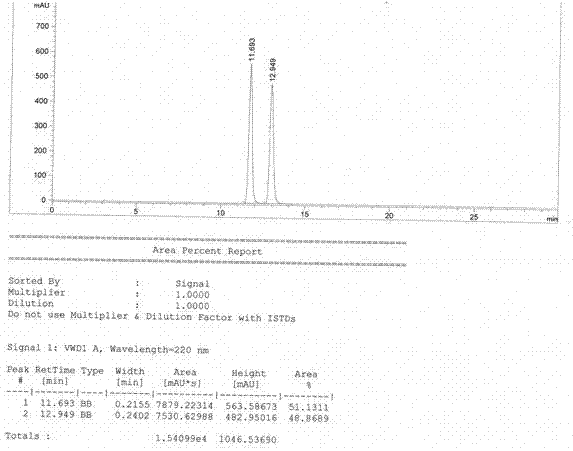
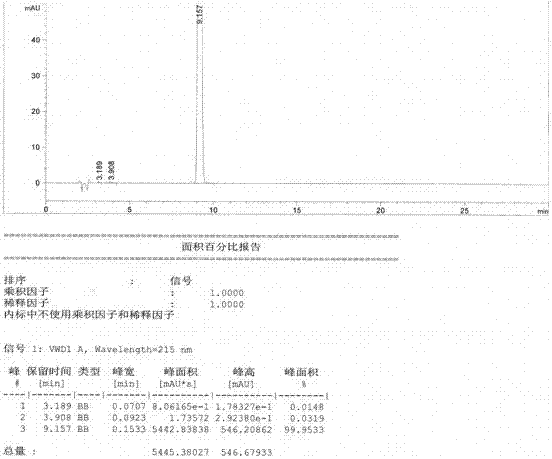
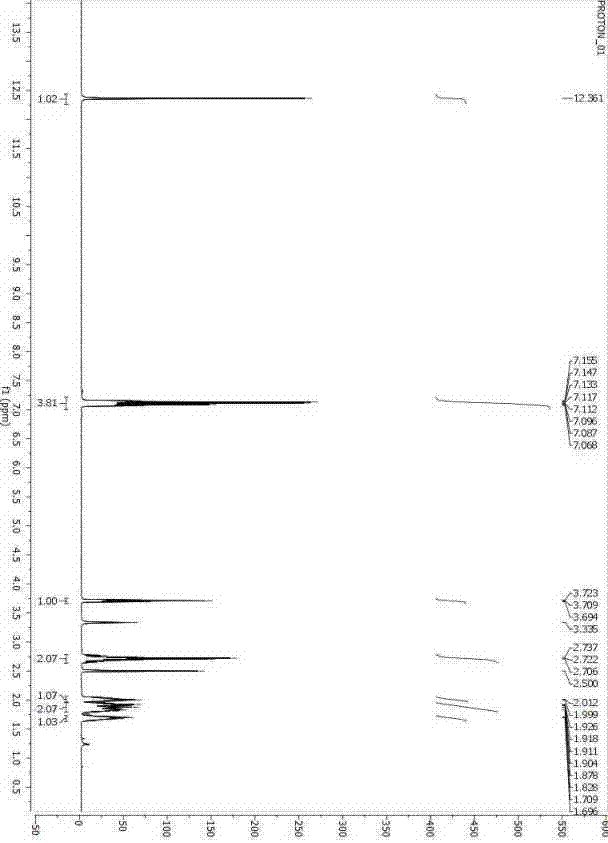
Method for preparing D-phenylalanine through dynamic kinetic resolution
InactiveCN102010345BHigh split yieldReduce operating costsOrganic compound preparationAmino-carboxyl compound preparationSolventOperating cost
The invention discloses a method for preparing D-phenylalanine through dynamic kinetic resolution. The method comprises the following steps of: reacting L-phenylalanine serving as raw materials with hydrochloric acid to generate L-phenylalanine hydrochloride; racemizing and resolving under the action of a solvent and a racemization catalyst by taking the L-phenylalanine hydrochloride as a substrate and dibenzoyl tartaric acid (L-DBTA) as a resolving agent to obtain D-phenylalanine.L-DBTA disalt; and finally performing triethylamine resolution to prepare the target product D-phenylalanine. Compared with the prior art, the method directly takes the L-phenylalanine as the resolving substrate, mild alcohol as the solvent and an aldehyde pyridine compound as the racemization catalyst, racemizes and resolves the L-phenylalanine into the D-phenylalanine.L-DBTA disalt, has a theoretical conversion rate of about 100 percent, greatly improves the resolution yield, reduces the operating cost, ensures stable product quality and is suitable for industrial production.
Owner:SHANGHAI HUAYI GRP CO
Method for preparing (S)-tetrahydro-1-naphthoic acid through high-efficiency resolution
InactiveCN107382697AReduce pollutionHigh split yieldPreparation from carboxylic acid saltsOptically-active compound separationNaphthaleneRacemization
The invention relates to a preparation method of a palonosetron hydrochloride key intermediate (S)-1,2,3,4-tetrahydro-1-naphthoic acid as shown in a formula (I). The method comprises the following steps of adding a resolution agent for resolution by adopting 1,2,3,4-tetrahydro-naphthalene acid racemate as a raw material to obtain a salt (III) formed by a (S)-1,2,3,4-tetrahydro naphthalene acid and the resolution agent, and a salt (IV) formed by (R)-1,2,3,4-tetrahydro naphthalene acid and the resolution agent; carrying out salt removing on a compound III and a compound IV separately to obtain compounds (I) and (II); and carrying out racemization on the compound (II) under an alkaline condition to obtain a starting material 1,2,3,4-tetrahydro-naphthalene acid racemate and further carrying out resolution according to the steps. Therefore, the overall yield can be greatly improved, the production cost is reduced and environmental protection is facilitated. The preparation technology of the palonosetron hydrochloride key intermediate provided by the invention is different from the prior art, is safe, environmentally friendly, simple in operation and high in yield, and has relatively great practical value. The formula is as shown in the specification.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
A kind of preparation method of levocloperastine fendizaic acid
The invention discloses a preparation method of levo cloperastine fendizoate, which comprises the following steps: carrying out nucleophilic substitution reaction on 4-chlorobenzhydrol and 2-chloroethanol in a benzene organic solvent, so that an intermediate product is obtained; reacting the intermediate product with piperidine, so that racemic cloperastine is obtained; resolving the racemic cloperastine by using a resolving agent in a fatty alcohol solvent, so that levo cloperastine is obtained; and carrying out salt forming reaction on the levo cloperastine and a fendizoic acid, so that levo cloperastine fendizoate is obtained, wherein the resolving agent is an R-substituted dibenzoyl-L-tartaric acid, and R refers to alkyl, alkoxy, -Cl, -F, -Br or -H. In the method provided by the invention, in a fatty alcohol solvent, an R-substituted dibenzoyl-L-tartaric acid is adopted as a resolving agent for carrying out resolving on racemic cloperastine, and the resolving yield is high, so that the obtained levo cloperastine fendizoate has high optical purity, and has a high product yield.
Owner:CHONGQING HENG AN CHEM CO LTD
Dexmedetomidine hydrochloride intermediate resolution method
InactiveCN106632053AOvercome the defect of low split efficiencyFavorable supply securityOrganic chemistry methodsFiltrationMarket potential
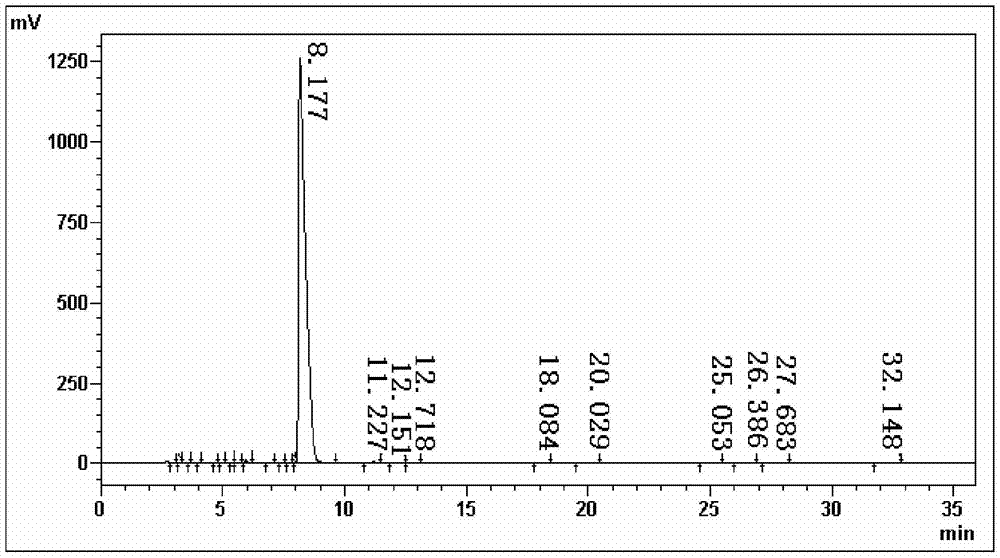
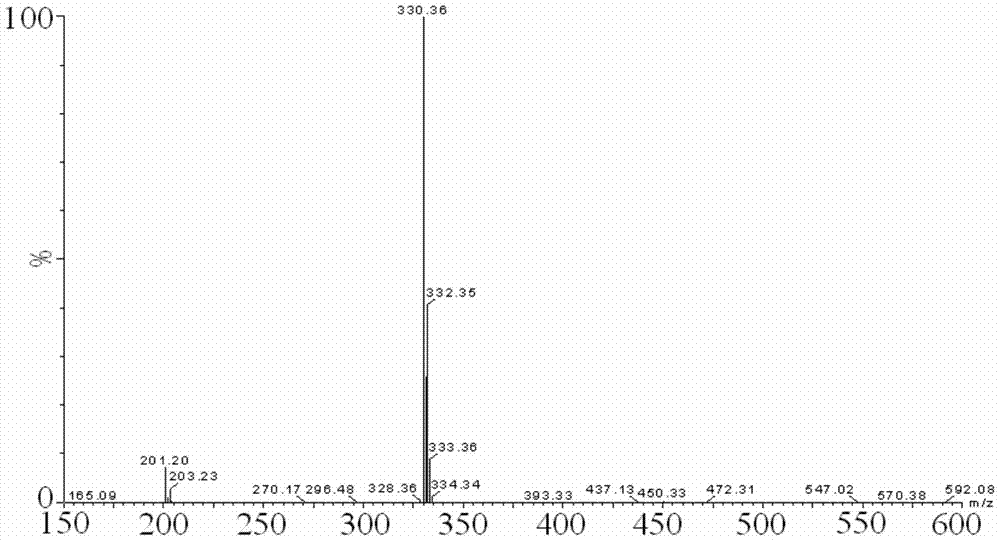
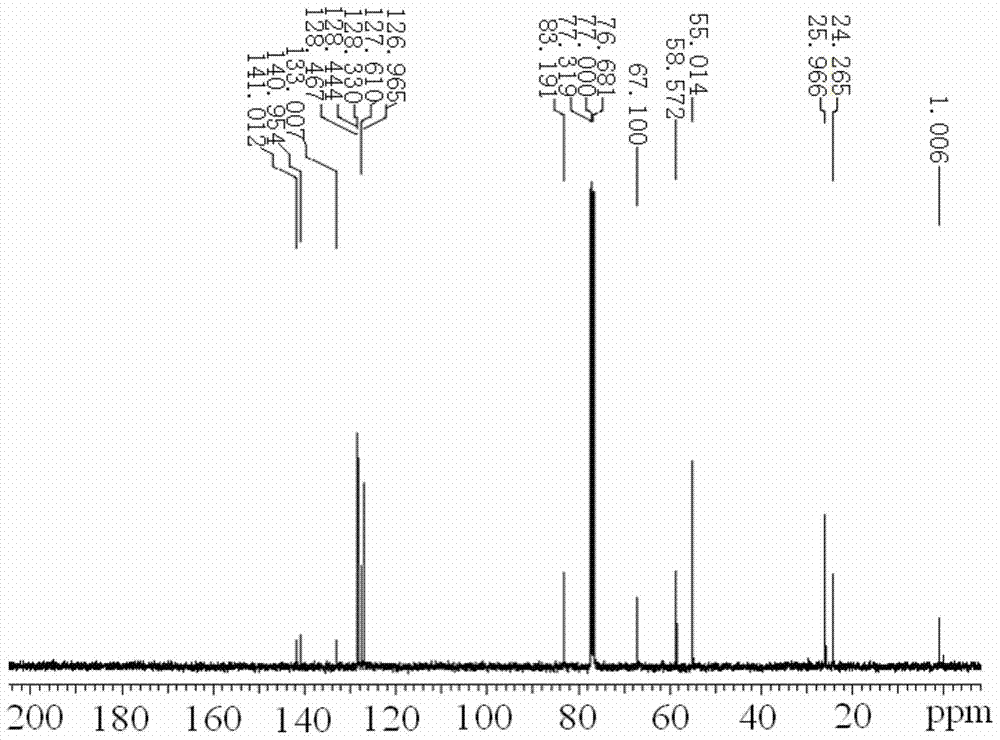
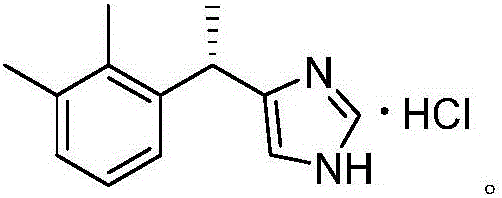
The invention discloses a dexmedetomidine hydrochloride intermediate resolution method. The resolution method includes: (1) subjecting salt racemized 4-[1-(2,3-dimethyl phenyl)ethyl]-1H-imidazole to sulfonation reaction with a sulfonating agent; (2) subjecting a product obtained in the step (1) to stirring salt-forming reaction with lysine ethyl ester in absolute ethyl alcohol, standing, and performing suction filtration to obtain (S)-salt; (3) allowing reaction of the (S)-salt obtained in the step (2) in sodium hydroxide aqueous solution to obtain (S)-4-[1-(2,3-dimethyl phenyl)ethyl]-1H-imidazole. According to the resolution method, sulfonation is performed prior to adoption of chiral compound lysine ethyl ester for resolution, high resolution efficiency and resolution yield can be achieved. By providing of the dexmedetomidine hydrochloride intermediate resolution method, a beneficial raw material supply guarantee can be provided for dexmedetomidine hydrochloride, and great market potential is realized.
Owner:XUZHOU MEDICAL UNIV
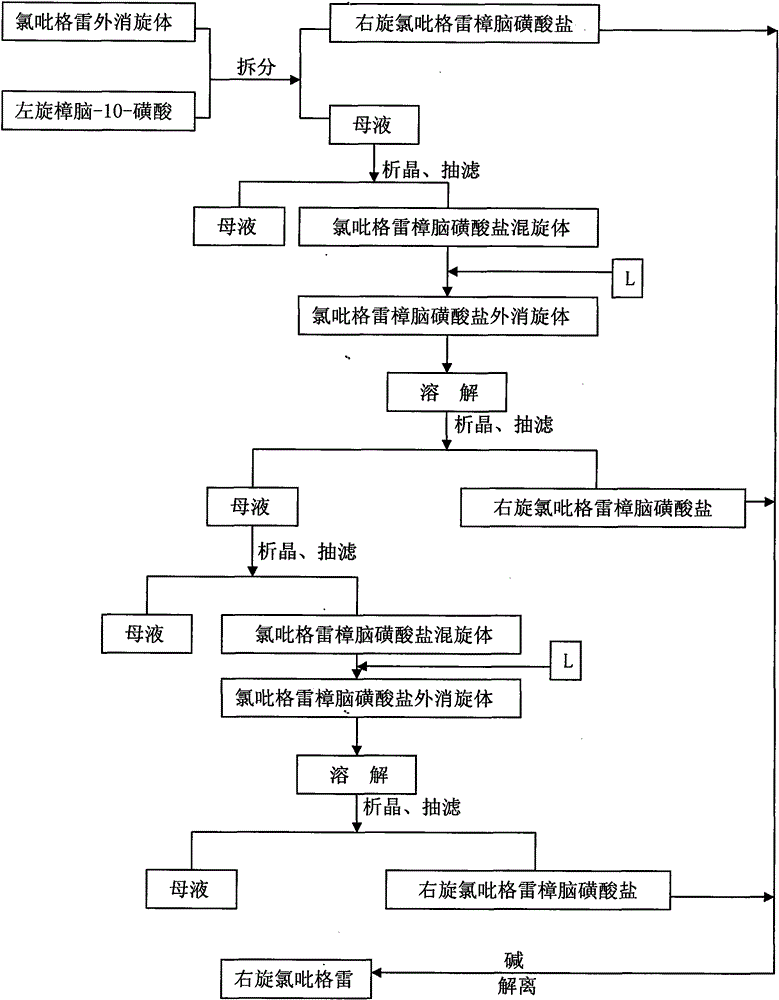
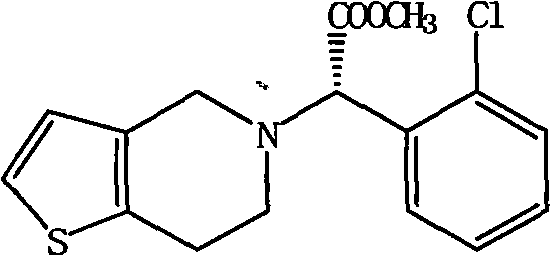
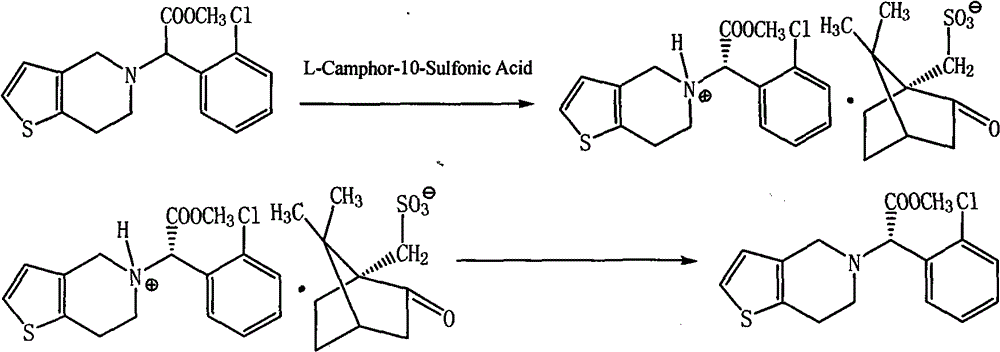
Method for improving resolution yield of clopidogrel camphorsulfonate
The invention discloses a synthesis method capable of improving the yield of dextro-clopidogrel camphorsulfonate. In the method, racemate of clopidogrel is used as a raw material, the dextro-clopidogrel camphorsulfonate is obtained by repeatedly performing the steps of resolution with the resolving agent, crystallization, vacuum filtration and drying, and the obtained dextro-clopidogrel camphorsulfonate is dissociated by a dissociating agent to obtain the dextro-clopidogrel camphorsulfonate with a yield of over 90 percent. The enantiomer of the clopidogrel dextroisomer product obtained by the method is less than 0.1 percent, and the ee value of the clopidogrel dextroisomer product obtained by the method is over 99.5 percent. Compared with other methods, the method has the advantage of simple operation.
Owner:CHANGZHOU PHARMA FACTORY
A kind of preparation method of rotigotine
ActiveCN113234057BReduce lossHigh split yieldOrganic compound preparationOrganic chemistry methodsTetraloneMandelic acid
Owner:CHENGDU TECH UNIV +1
Preparation method of enclomiphene
InactiveCN107033013AImprove liquidityEasy to recycleOrganic compound preparationAmino-hyroxy compound preparationAlcoholEnclomiphene
The invention relates to a preparation method of enclomiphene. The preparation method comprises steps as follows: (1) clomiphene or salt thereof and racemic 1,1'-binaphthyl-2,2'-diyl hydrogenphosphate are subjected to a reaction in alcohol solvents containing ether solvents, and an enclomiphene and 1,1'-binaphthyl-2,2'-diyl hydrogenphosphate compound in a solid form is obtained; (2) the enclomiphene and 1,1'-binaphthyl-2,2'-diyl hydrogenphosphate compound in a solid form is subjected to a free reaction, and enclomiphene is prepared. With the alcohol solvents containing the ether solvents as splitting solvents, enclomiphene and the splitting solvents can form a solid compound which is directly separated out from the splitting solvents, the flowability of a reaction system is improved, and the enclomiphene preparation method with better industrial application prospects is provided, and by means of the preparation method, the splitting yield is high, the purity of a split product is high, the cost is low and environmental pollution is low.
Owner:SHANGHAI DUDE MEDICAL SCI & TECH CO LTD +1
Synthesis and resolution of n-[4-(1-aminoethyl)-2,6-difluorophenyl]methanesulfonamide
ActiveCN109608368BReduce pollutionHigh purityOrganic compound preparationOrganic chemistry methodsHydroxylamineEthyl group
The invention discloses a synthesis and resolution method of N-[4-(1-aminoethyl)-2,6-difluorophenyl]methanesulfonamide. The synthesis method comprises 3,4,5-trifluorobenzene Ethanone reacts with methanesulfonamide to obtain N-(4-acetyl-2,6-difluorophenyl) methanesulfonamide, and then reacts with hydroxylamine hydrochloride to obtain N-[4-(hydroxylaminoethyl)-2, 6‑difluorophenyl] methanesulfonamide, finally obtained by catalytic hydrogenation. The resolving agent adopted in the resolution method is D-mandelic acid, etc., the solvent used is water, etc., and the resolution temperature is 40-50°C. The synthesis method of the invention has simple operation, low production cost, less environmental pollution, high product purity and high reaction yield, and is suitable for large-scale industrial production. In the resolution method of the present invention, a higher resolution yield and optical purity can be finally obtained by selecting a suitable resolution agent, solvent, resolution temperature, especially the addition method of the resolution agent.
Owner:CHANGZHOU SUNLIGHT PHARMA
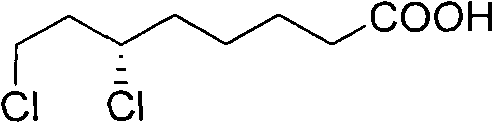
Method for splitting 6,8-dichlorocaprylate
InactiveCN101880224AReduce pollutionReduce consumptionCarboxylic compound separation/purificationSolubilityDiastereomer
The invention provides a method for optically splitting (+ / -)-6,8-dichlorocaprylate. In the method, dehydroabietylamine with a structure expressed as the following figure is used as a splitting reagent, the dehydroabietylamine is reacted with racemized (+ / -)-6,8-dichlorocaprylate to form a pair of diastereoisomer salts, and optical splitting is performed according to different solubility of the diastereoisomer salts to obtain (+)-6,8-dichlorocaprylate. Compared with the conventional splitting method, the splitting reagent is cheap and easily obtained, and the product is purified without repeated recrystallization.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Enzymatic resolution method of isavuconazole intermediate
ActiveCN105907832AHigh split yieldThe product has high chiral purityFermentationSide productEnantiomer
The invention provides an enzymatic resolution method of an isavuconazole intermediate. Enantiomer impurities in a nitrilase (2R, 3S)-isavuconazole intermediate I is hydrolyzed to form a (2S, 3S)-carboxylic acid side product and the (2S, 3S)-carboxylic acid side product is separated and purified to form an optically pure (2S, 3R)-isavuconazole intermediate I.
Owner:CHENGDU NORMAL UNIV
Spliting method for DL-pantoyl intenral ester
InactiveCN1199961CHigh selectivityImprove overall utilizationOptically-active compound separationOrganic racemisationOrganic solventOrganic base
Owner:ZHEJIANG NHU CO LTD
Method for preparing D-serine by kinetic resolution
ActiveCN101735085BHigh split yieldReduce operating costsOrganic compound preparationAmino-carboxyl compound preparationSerine methyl esterKinetic resolution
The invention discloses a method for preparing D-serine by kinetic resolution. The method takes DL-serine as raw material and is implemented by esterifying DL-serine with methanol under catalysis of Amberlyst-15 ion exchange resin to obtain DL-serinemethylester, carrying out dynamic kinetic resolution on DL-serinemethylester and resolving agent L-DBTA under action of racemization catalyst to obtain dibasic DL-serinemethylester.L-DBTA, dissociating with hydrochloric acid, and hydrolyzing to obtain D-serine, the target product of the invention. Compared with the prior art, by using the racemization catalyst in the invention, the dynamic continuous conversion of dibasic L-serinemethylester.L-DBTA into dibasic DL-serinemethylester.L-DBTA can be realized with the theoretic conversion rate being approximate to 100%, thus greatly enhancing yield of resolution, reducing operation cost, stabilizing product quality and being suitable for industrialized production.
Owner:SHANGHAI SHISI CHEM PROD
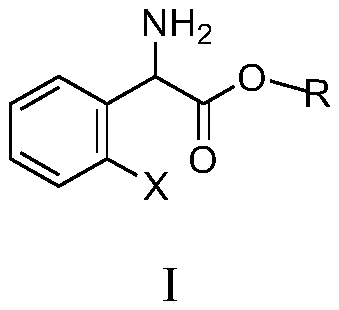
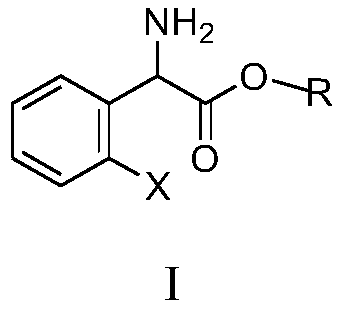
Substituted aromatic ring phenylglycine fatty alcohol ester resolution method
InactiveCN109734617ATake advantage ofSimple processOrganic compound preparationAmino-carboxyl compound preparationFiltrationReaction temperature
The invention discloses a substituted aromatic ring phenylglycine fatty alcohol ester resolution method which includes the steps: dissolving resolution agents in proper solvents by stirring; adding aldehyde or ketone accounting for 0.1-10% of the weight of a compound as shown in a formula I and serving as a catalyst; dropping mixed solution formed by dissolving the compound as shown in the formulaI in the proper solvents; performing reaction for 1-48 hours at the temperature of 20-50 DEG C; cooling the solution after complete reaction; performing suction filtration and drying to obtain S-(+)-substituted aromatic ring phenylglycine fatty alcohol ester salt. The method has the advantages that a technology is simple and convenient, a delta Rf value serves as a separation index for orthogonal experiments to rapidly screen resolution solvents, yield and an ee value serve as indexes for orthogonal experiments to rapidly screen resolution catalysts, the yield is high and reaches 93%, operation is simple and convenient, reaction time is short, reaction temperature is low, the solvents and the resolution agents are recycled and can be sufficiently used, and the method is environmentally friendly.
Owner:EAST CHINA UNIV OF SCI & TECH
A method for splitting dl-2-aminopropanol to prepare 1-2-aminopropanol
InactiveCN106810458BIncrease profitHigh split yieldOrganic compound preparationOrganic chemistry methodsDistillationFiltration
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Resolution process of racemic amino pentanamide
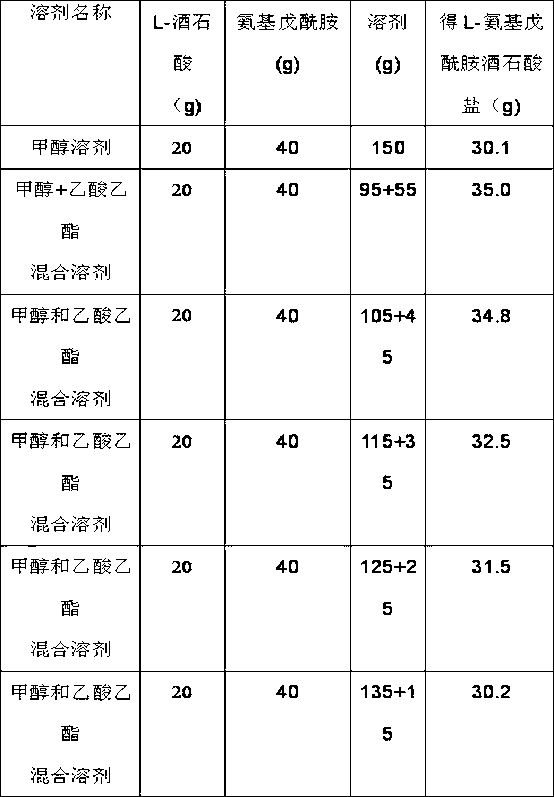
InactiveCN102827029AHigh split yieldCarboxylic acid amides optical isomer preparationAcetic acidResolution rate
The invention relates to a resolution process of racemic amino pentanamide, which comprises the following steps that methanol and ethyl acetate are prepared into a solvent mixture according to a mass ratio of 1:2 to 2.5, and then L-tartaric acid is dissolved into the solvent mixture according to a mass ratio of 1:1 7. 5 to 8; the temperature is reduced to below 10 DEG C, and the racemic amino pentanamide is slowly added according to a mass ratio of racemic amino pentanamide to L-tartaric acid of 2 to 2.5:1; and after addition, the reaction is continuously carried out for 2 to 2.5 hours, a material is discharged and filtered to obtain a filter cake which is the resolution product L-amino pentanamide tartrate. With the resolution process, the experimental data shows that the resolution rate can be increased by 15%.
Owner:ZHEJIANG BANGCHENG CHEM
A kind of method of splitting isavuconazole intermediate by enzymatic method
Owner:CHENGDU NORMAL UNIV
New benzocyclobutane, preparation method thereof and application thereof
InactiveCN101671265BEasy to getMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationIVABRADINE HYDROCHLORIDECombinatorial chemistry
The invention provides a preparation method for (1S)-4, 5-dimethoxy-1-(methyl-amino-methyl)-benzocyclobutane serving as a key intermediate of ivabradine hydrochloride and addition salt thereof, and meanwhile provides a method for preparing an intermediate of the (1S)-4, 5-dimethoxy-1-(methyl-amino-methyl)-benzocyclobutane and a method for splitting the intermediate.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Optical resolution method substituting oxyphosphonate acetate
InactiveCN100497335CHigh optical purityReduce consumptionGroup 5/15 element organic compoundsAcetic acidBenzene
This invention provides a optical resolution preparation that substitutes oxygen phosphino-acetic acid, concretely relating to optical resolution preparation of racemoid which consists of [(R)-[(1S)-2-methyl-1-(1-keto-propoxy) propoxy] ( 4-benzene butyl)oxygen phosphino]acetic acid and [(S)-[(1R)-2-methyl-1- (1-keto-propoxy)propoxy](4- benzene butyl)oxygen phosphino]acetic acid. The preparation of this invention uses 10,11-di-H Cinchonidine shown in following formula(1) as resolution agent, compared with present resolution preparation, the yield and optical purity of resolution is obviously raised, it need not recryst many times to depurate, and dosis of resolution agent is reduced.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com