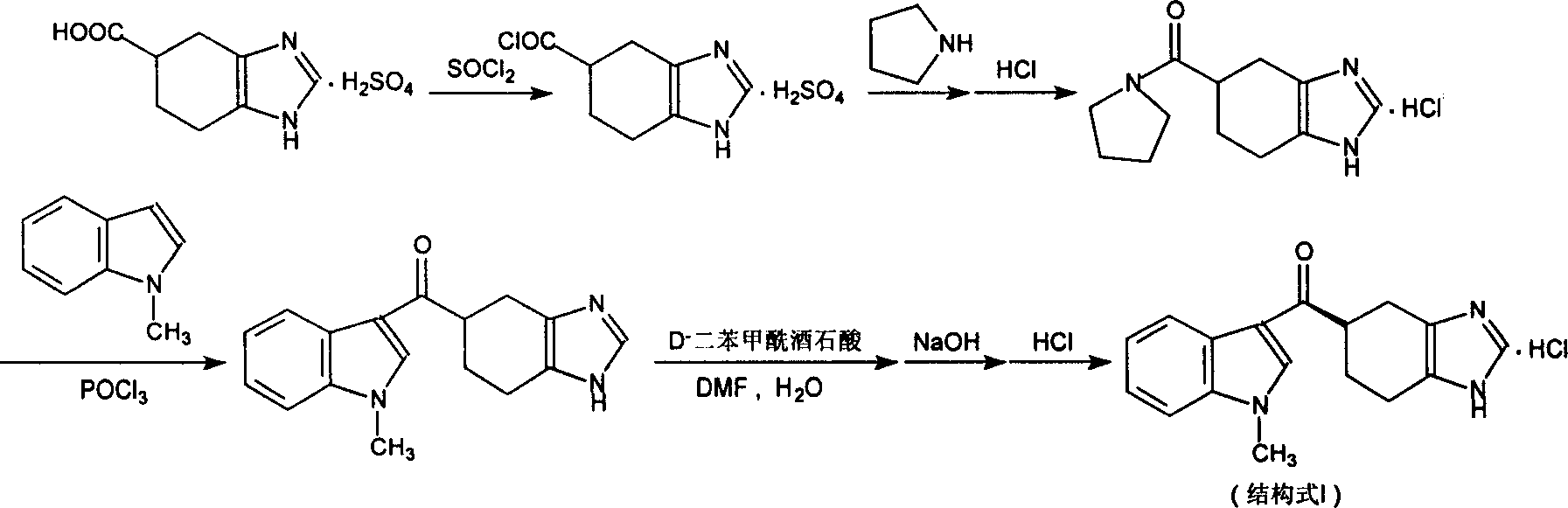
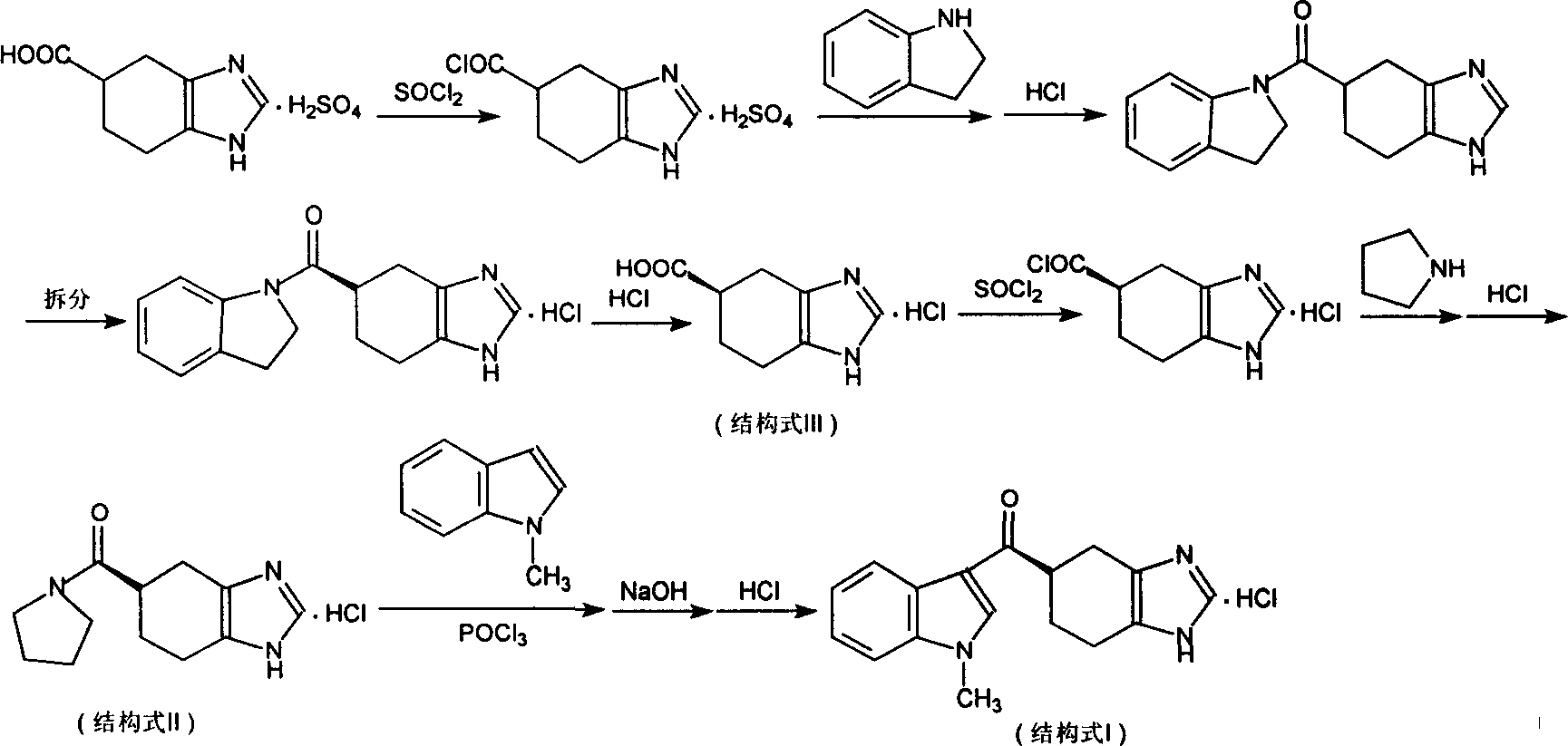
Novel preparation method of ramosetron hydrochloride
A technology of ramosetron and structural formula, which is applied in the field of medicinal chemistry, can solve the problems of difficult solvent recovery, large consumption of side chain N-methyl indole, high cost, etc., and achieves reduced consumption of N-methyl indole and facilitates recovery The effect of reuse and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of 5-formyl chloride-4,5,6,7-tetrahydro-1H-benzimidazole sulfate (method A)
[0032] Suspend 43.4 g (0.20 mol) of 5-formic acid-4,5,6,7-tetrahydro-1H-benzimidazole sulfate in 500 ml of dichloroethane, add 47.6 g (0.40 mol) of thionyl chloride, Stir, heat up to 60°C, keep the temperature for reaction for 2 hours, evaporate the solvent under reduced pressure to obtain 36.6 g of crude product of 5-formyl chloride-4,5,6,7-tetrahydro-1H-benzimidazole sulfate, the yield : 77.7%, which can be directly used in the following reaction without further refining.
Embodiment 2
[0033] Example 2: Preparation of 5-formyl chloride-4,5,6,7-tetrahydro-1H-benzimidazole sulfate (method B)
[0034] 5-formyl chloride-4,5,6,7-tetrahydro-1H-benzimidazole sulfate can also be directly reacted with a large excess of thionyl chloride, and the specific method is to prepare 5-formic acid-4,5, 43.4g (0.20mol) of 6,7-tetrahydro-1H-benzimidazole sulfate was suspended in 238g (2.0mol) of thionyl chloride, stirred, heated to reflux, reacted for 2 hours, concentrated under reduced pressure to recover excess Thionyl chloride obtained 42.8g of 5-formyl chloride-4,5,6,7-tetrahydro-1H-benzimidazole sulfate crude product, yield: 90.9%, and it does not need to be further refined, and can be directly used in the following Reaction.
Embodiment 3
[0035] Example 3: Preparation of 5-formyl chloride-4,5,6,7-tetrahydro-1H-benzimidazole hydrochloride
[0036]Referring to Example 1 or 2, replace 5-formic acid-4,5,6,7-tetrahydro-1H-benzene with 5-formic acid-4,5,6,7-tetrahydro-1H-benzimidazole hydrochloride And imidazole sulfate, the resulting crude product can be directly used in the following reaction without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com