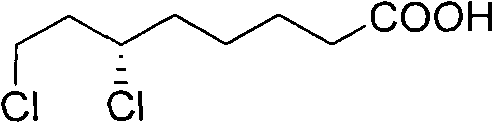
Method for splitting 6,8-dichlorocaprylate
A technology of dichlorooctanoic acid and a splitting agent, which is applied in the splitting field of 6,8-dichlorooctanoic acid, can solve the problems of high price of ephedrine, unsuitable for splitting agent, complicated operation, etc. The effect of splitting yield and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
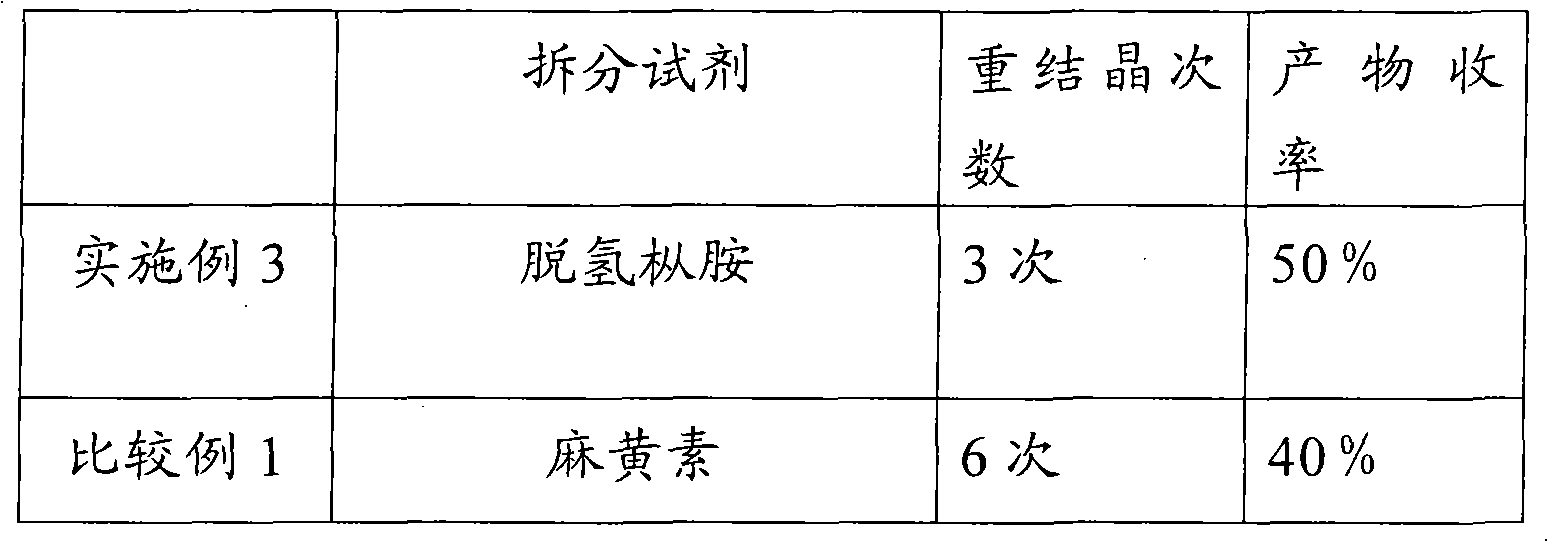
[0027] In five identical 50ml single-necked flasks, add 5g (23.5mmol) (±)-6,8-dichlorooctanoic acid and 20ml ethyl acetate respectively, shake well, and then add 18.8mmol of resolution reagent cinchor Nidine, quinine, dehydroabietylamine, L-aminopropanol, cinchonine, stirred in a water bath at 40°C until clear. Slowly cool to 0 DEG C then, observe phenomenon and find, only add the resolving agent dehydroabietylamine in the flask that precipitates crystal, add in the flask that adds other resolving reagent or clarification does not have phenomenon, or separates out oily thing.
Embodiment 2
[0029] In a 50ml single-necked flask, add 2g (9.4mmol) (±)-6,8-dichlorooctanoic acid and 8ml ethyl acetate, shake well, then add 1.9g (6.6mmol) dehydroabietylamine, and Stir in a water bath until clear. Then it was slowly cooled to 0°C, and crystals were formed. After suction filtration, the collected crystals were recrystallized three times in ethyl acetate, and dried in vacuo to obtain 1.1 g of a white solid, which was a salt of (+)-6,8-dichlorooctanoic acid and dehydroabietylamine. Rotation, [α] 20 D: -11.2° (C=1, ethanol).
Embodiment 3
[0031] In a 100ml single-necked flask, add 8.8g (41.3mmol) (±)-6,8-dichlorooctanoic acid and 36ml ethyl acetate, shake well, then add 9.4g (33.1mmol) dehydroabietylamine, at 40 ℃ Stir in a water bath until clear. Then it was slowly cooled to 0°C, and crystals were formed. Suction filtration, the collected crystals were recrystallized three times in ethyl acetate, and dried in vacuo to obtain 5.2 g of a white solid, which was a salt of (+)-6,8-dichlorooctanoic acid and dehydroabietylamine, [α] 20 D: -12° (C=1, ethanol). The above white solid was basified with NaOH first, and the separated alkali solution was acidified with dilute hydrochloric acid to free (+)-6,8-dichlorooctanoic acid, then extracted with dichloromethane, dried, and concentrated to obtain an oil (+)-6,8-dichlorooctanoic acid. )-6,8-dichlorooctanoic acid 2.2g. Rotation, [α] 20 D: +30.0° (c=2, toluene)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com