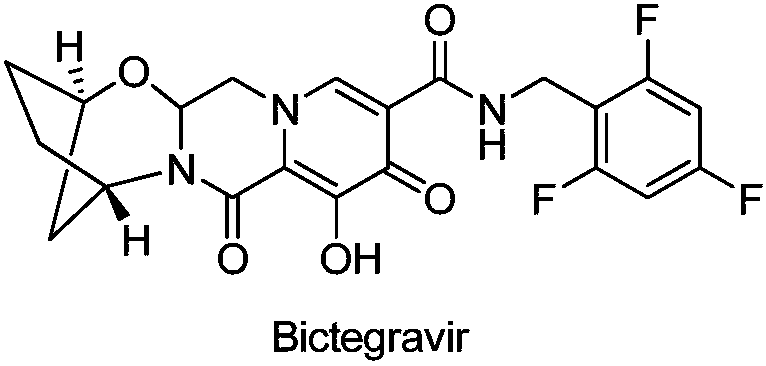
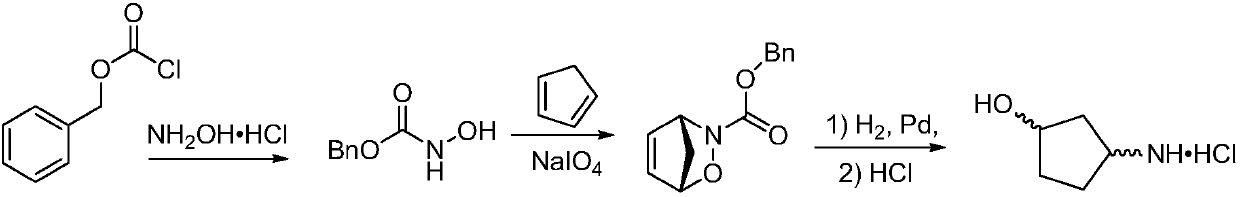
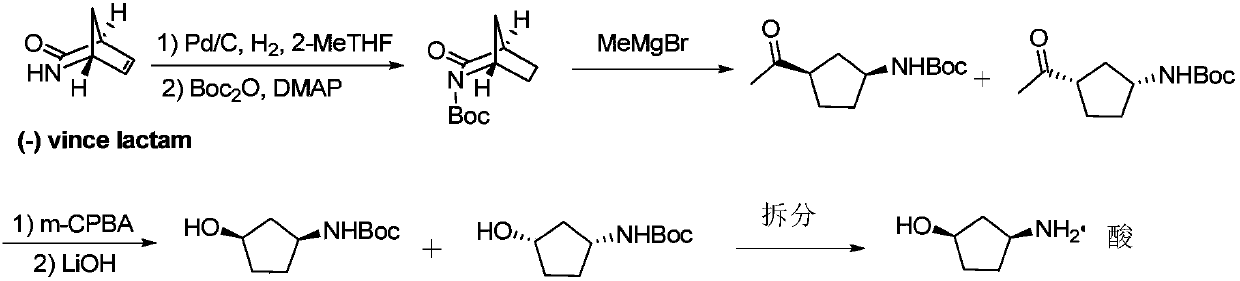
Method for preparing single-configuration 3-aminocyclopentanol through chiral resolution
A technology for the separation of aminocyclopentanol and chirality, which is applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as high cost, low optical purity, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] The cis-3-aminocyclopentanol racemic mixture (50 g, 0.495 mol) was dissolved in 400 mL of isopropanol, and the resolution reagent D-tartaric acid (59.4 g, 0.396 mol) was added, stirred at room temperature, and a large amount of solids precipitated. After continuing to stir for 1 to 2 hours, filter with suction, and rinse the filter cake once with isopropanol to obtain crude (1R,3S)-3-aminocyclopentanol tartrate as a white solid, with a wet weight of 80.76 g and an ee value of 92.1%. .
[0074] Suspend the crude product of (1R,3S)-3-aminocyclopentanol tartrate obtained above in 300ml of 95% ethanol, raise the temperature to reflux to dissolve the liquid, slowly lower the temperature to room temperature, and further cool down to 10-15°C, keep stirring After 1 to 2 hours, filter to obtain a refined solid, and its ee value is measured to be 98.5%.
[0075] Repeat the refining step to obtain 52.4 g of refined (1R,3S)-3-aminocyclopentanol tartrate, and its ee value is 99.2%....
Embodiment 2
[0077] The cis-3-aminocyclopentanol racemic mixture (50 g, 0.495 mol) was dissolved in 400 mL of absolute ethanol, and the resolution reagent D-tartaric acid (74.2 g, 0.495 mol) was added, stirred at room temperature, and a large amount of solids precipitated. After continuing to stir for 1 to 2 hours, filter with suction, and rinse the filter cake once with absolute ethanol to obtain (1R,3S)-3-aminocyclopentanol tartrate as a white solid crude product, with a wet weight of 82.3g and an ee value of 91.2%. .
[0078] Suspend the crude product of (1R,3S)-3-aminocyclopentanol tartrate obtained above in 300ml of absolute ethanol, raise the temperature to reflux to dissolve the liquid, slowly lower the temperature to room temperature, and further cool down to 10-15°C, keep stirring After 1 to 2 hours, filter to obtain a refined solid whose ee value was measured to be 98.6%.
[0079] The above refining steps were repeated to obtain 53.0 g of (1R,3S)-3-aminocyclopentanol tartrate af...
Embodiment 3
[0082] Dissolve cis-3-aminocyclopentanol racemic mixture (50g, 0.495mol) in 400mL of 95% ethanol, add resolution reagent D-di-p-toluoyl tartaric acid (152.8g, 0.396mol), heat up to 40°C and keep stirring After 2 hours, a large amount of solid precipitated. After lowering to room temperature and continuing to stir for 1-2 hours, suction filtration, the filter cake was rinsed once with 95% ethanol to obtain white solid (1R,3S)-3-aminocyclopentanol tartrate, wet weight 140.2g, ee value 92.1%.
[0083] Suspend the crude product of (1R,3S)-3-aminocyclopentanol tartrate obtained above in 400ml of 95% ethanol, raise the temperature to reflux to dissolve the liquid, slowly lower the temperature to room temperature, and further cool down to 10-15°C, keep stirring After 1 to 2 hours, filter to obtain a refined solid whose ee value was measured to be 94.3%.
[0084] The above refining steps were repeated twice to obtain 48.8 g of (1R,3S)-3-aminocyclopentanol di-p-toluoyl tartrate after...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com