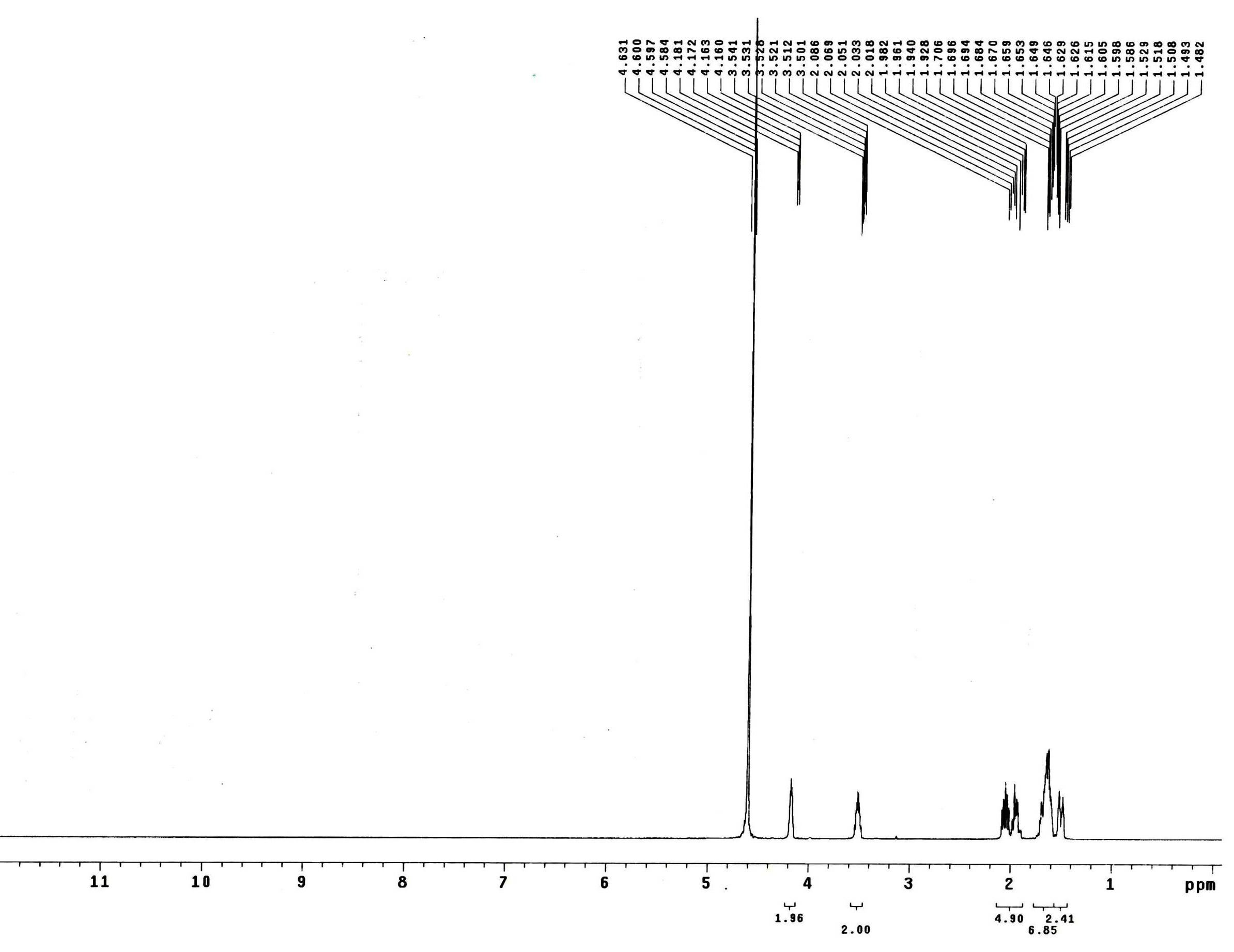
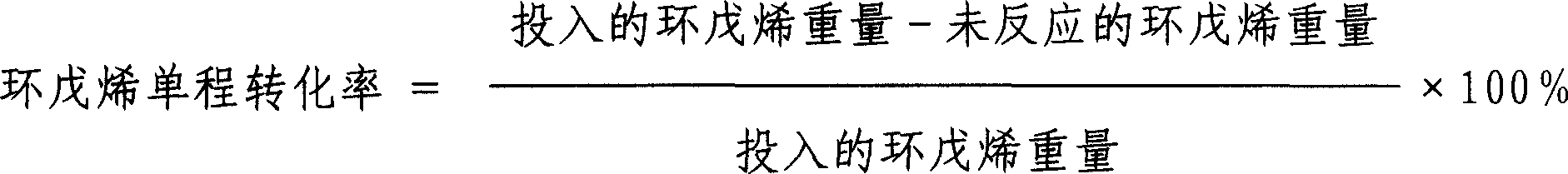
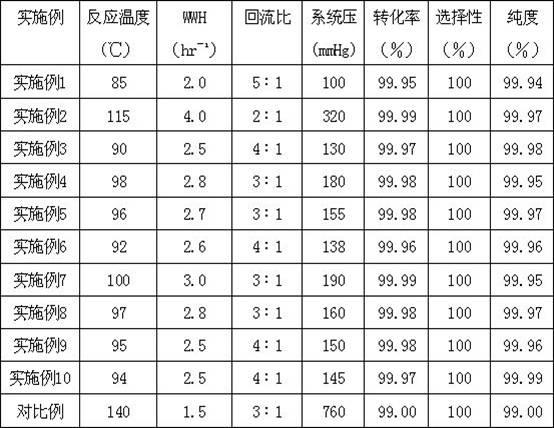
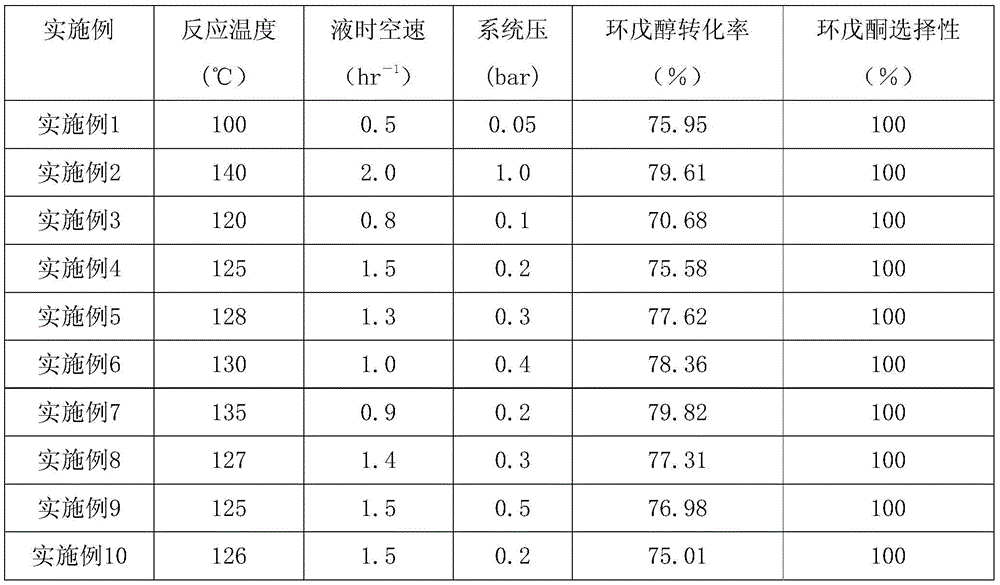
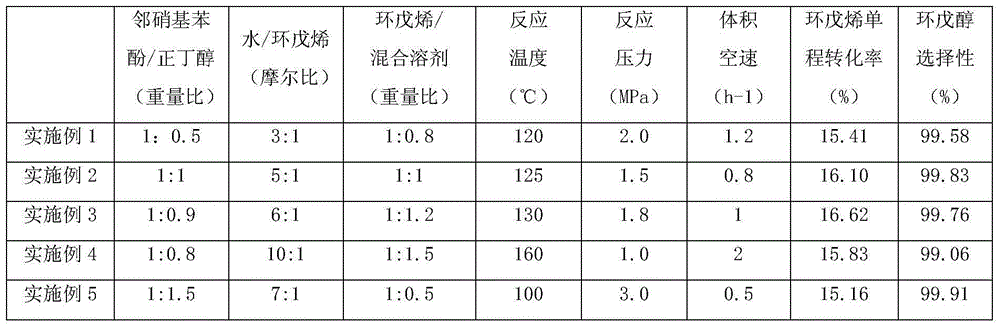
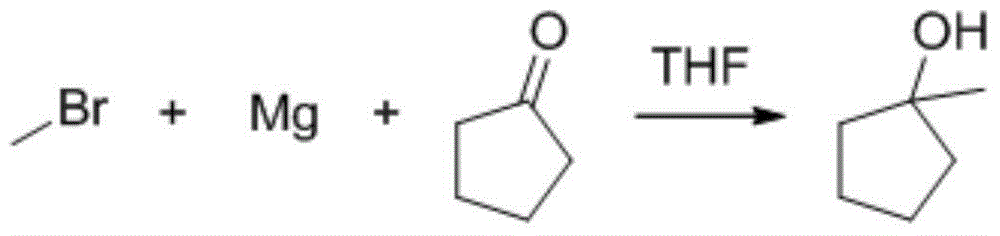Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
161 results about "Cyclopentanol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclopentanol or cyclopentyl alcohol is a cyclic alcohol. It is also known as hydroxycyclopentane.
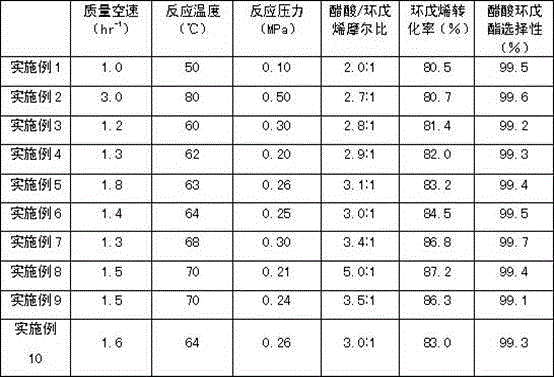
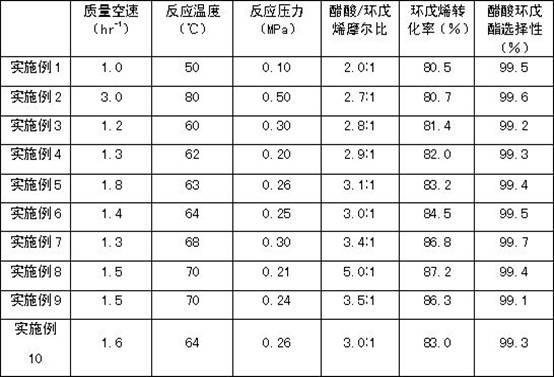
Method for preparing cyclopentanone and/or cyclopentanol by furfural or furfuryl alcohol
InactiveCN102807483AIncrease productionImprove conversion rateOrganic compound preparationHydroxy compound preparationAlcoholCyclopentanol
The invention provides a method for preparing cyclopentanone and / or cyclopentanol by furfural or furfuryl alcohol. The method includes the following steps of firstly, mixing furfural or furfuryl alcohol with solvent according to proportion; secondly, adding catalyst with hydrogenating function into the mixture in the first step; and thirdly, reacting in the reducing atmosphere to obtain cyclopentanone and / or cyclopentanol. The method for preparing cyclopentanone and / or cyclopentanol by furfural or furfural alcohol is high in yield of the cyclopentanone and / or cyclopentanol, and further, is simple in process, convenient to operate, mild in reaction conditions, no carbon deposition is caused during the whole reaction, the catalyst is low in cost and easy to obtain and can be reused without lowering activity.
Owner:UNIV OF SCI & TECH OF CHINA
Cyclopentanol preparing and refining method
ActiveCN1676504AEasy to refineHigh selectivityOrganic compound preparationPreparation by hydroxy group additionHydration reactionCyclopentene
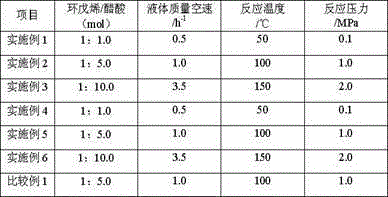
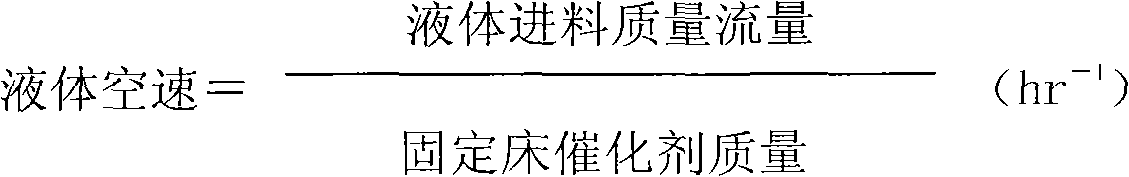
This invention is a making and refining method of cyclopentanol, which includes: 1) the materials comprises cyclopentene, phenylic acid solvent, water activator continuously pass through catalyst fixed bed to carry out hydration reaction, and the volume space velocity is 2-15hr to the power -1, the mole ratio of cyclopentene and water is 0.8-5.0, the weight ratio of phenylic acid and cycloamylene is 0.5-1.0. the intensity of activator is 0.01-0.2wt%, the reaction temperature is 130-180deg.C, the reaction pressure is 1.0-3.0MPa, the catalyst is strong acid cation exchange resin with sulfo group anchored on the surface, and the activating solvent is trialkylamine; 2)the reacted materials cool to house temperature and demix into oil phase and aqueous phase, the oil phase materials through continuous distilling separation procedure get non-reacting cycloamylene, refined cyclopentanol and phenylic acid full of solvent. The strong points of this invention are that the conversion of cyclopentene and selectivity of cyclopentanol are high, and the refining of cyclopentanol is simple and low energy consumption.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Method for preparing cyclopentanol by hydrating cyclopentene
ActiveCN106674003AImprove conversion rateHigh selectivityOrganic compound preparationCarboxylic acid esters preparationCyclopenteneAcetic acid
The invention discloses a method for preparing cyclopentanol by hydrating cyclopentene. The method comprises the following steps: (1) performing addition reaction on cyclopentene and acetic acid under the action of modified sulfonic acid group cation exchange resin to produce cyclopentyl acetate, wherein the modified sulfonic acid group cation exchange resin is prepared by soaking the conventional sulfonic acid group cation exchange resin with methylbenzene and methyl isobutyl ketone sequentially; (2) enabling the material obtained in the step (1) to enter a rectifying tower, forming azeotrope with water on the lower part of the rectifying tower, performing hydrolysis reaction on the material and water under the action of a sulfonic acid group cation exchange resin catalyst filling the upper part of the rectifying tower, withdrawing a product cyclopentanol from the top of the tower and withdrawing acetic acid from the bottom of the tower. The method has the advantages of simple technical process, low energy consumption, high conversion rate, high selectivity, high product yield and the like.
Owner:CHINA PETROLEUM & CHEM CORP +1
Catalyst for catalyzing furfural or furfuryl alcohol to prepare cyclopentanol through hydrogenation rearrangement and preparation method and application method thereof
ActiveCN104998659AEasy to operateReduced activityOrganic compound preparationHydroxy compound preparationActive componentCyclopentanol
The invention provides a catalyst for catalyzing furfural or furfuryl alcohol to prepare cyclopentanol through hydrogenation rearrangement. The catalyst is a load type catalyst and comprises one or two active components and carriers; the one or two active components are selected from Pt or Pd or Ru or Rh or Cu or Ni; the carriers are one or two oxides which are selected from Co or Al or Mn or Ce; the mass of the active components is 0.5-8.0% of that of the carriers. According to the catalyst, the high-yield cyclopentanol can be obtained under the condition of moderate water phase hydrogenation by taking the furfural or the furfuryl alcohol as raw materials, the yield can reach 80 percent, and a continuable, low-cost, moderate and effective method is supplied to production of the important fine chemical cyclopentanol.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
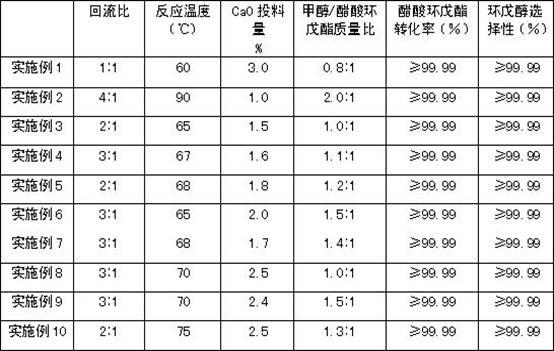
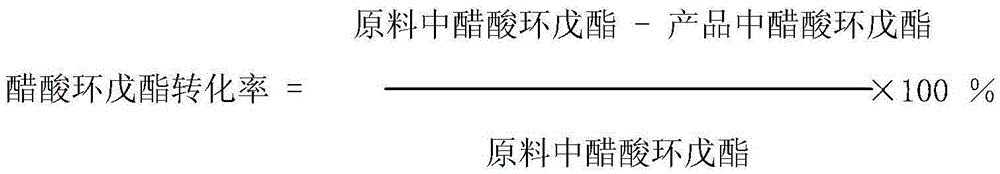
Method for preparing cyclopentanol from cyclopentene through indirect hydration method
ActiveCN102399133AEasy to recycleNo pollution in the processPreparation by alcoholysisProcess systemsCyclopentene
The invention relates to a method for preparing cyclopentanol from cyclopentene through an indirect hydration method. The method comprises steps that: liquid phase cyclopentene and acetic acid are subject to an addition reaction with a fixed-bed catalyst, such that cyclopentyl acetate is obtained, wherein the catalyst is sulfonic cation exchange resin with a mass exchange capacity of 3-5.5mmol / g;the product of the addition reaction is processed through rectification separation, such that refined cyclopentyl acetate is obtained; the refined cyclopentyl acetate is subject to an ester exchange reaction with methanol under the existence of a catalyst CaO, such that cyclopentanol and methyl acetate are produced; the produced methyl acetate is removed during the reaction process; the product of the ester exchange reaction is filtered, such that the catalyst is removed; and the obtained product is processed through rectification separation, such that the product cyclopentanol is obtained. The method provided by the invention provides substantial positive effects. The addition reaction and the ester exchange reaction have relatively high conversion rate and selectivity, and an average overall yield reaches approximately 80%. More importantly, no substance with high acidity and corrosivity is appeared in the reaction system during the whole reaction process. The raw materials which are not reacted can easily be recovered and reused, and almost no environment pollution is caused.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method of cis-3-amino-cyclopentanol hydrochloride
InactiveCN102633657AReduce consumptionReduce consumption and reduce production costsOrganic compound preparationAmino-hyroxy compound preparationCyclopentanolMedicinal chemistry
The invention discloses a preparation method of cis-3-amino-cyclopentanol hydrochloride, which comprises the steps of: (1) preparing a cis-3-amino-cyclopentanol intermediate A; (2) preparing a cis-3-amino-cyclopentanol intermediate B; (3) preparing a cis-3-amino-cyclopentanol crude product; (4) preparing a cis-3-amino-cyclopentanol hydrochloride crude product; and (5) obtaining the final product. The preparation method of the cis-3-amino-cyclopentanol hydrochloride has the advantages of being high in yield and purity, low in cost, safe and free from pollution.
Owner:甘肃科瑞生物科技有限公司
Method for preparing cyclopentanol by hydration of cyclopentene
ActiveCN1676506AImprove conversion rateHigh selectivityPreparation by hydroxy group additionHydration reactionCyclopentene
This invention is a method that cyclopentene makes cyclopentanol through hydration. It uses cyclopentene and water as raw materials, and cyclopentene, water and activator continuously pass through catalyst fixed bed to carry out hydration reaction, the mole ratio of cyclopentene and water is 0.8-5.0, reaction temperature 130-180deg.C, reaction pressure 1.0-3.0MPa, and the catalyst is strong acid cation exchange resin with sulfo group anchored on the surface, and the activating solvent is trialkylamine. The weigh ration of activators is: cycloamylene: activator=100:(0.2-5). This invention advances the conversion of hydration cyclopentene and maintains the selectivity at high level.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Method for preparing cyclopentanone through cyclopentanol dehydrogenation
InactiveCN102603506AImprove efficiencyReduce energy consumptionCarbonyl compound preparation by oxidationGas phaseDehydrogenation
The invention relates to a method for preparing cyclopentanone through cyclopentanol dehydrogenation. The method comprises the steps of carrying out catalytic dehydrogenation and rectification reactions on the raw material cyclopentanol so as to directly obtain the high-purity cyclopentanone, wherein the catalytic dehydrogenation temperature is 85-115 DEG C, the system pressure is 100-300mmHg, and granular Raney nickel type metal alloy comprising 53% of Al, 44% of Ni and 3% of Mo is adopted as a catalyst in the dehydrogenation reaction; and the cyclopentanol load WWH of the catalyst is 2-4hr-1. The gas-phase dehydrogenation reaction product directly enters in a rectifying column of which the number of theoretical plates is 45 to be purified and the reflux ratio of the rectifying column is controlled to be (2:1)-(5:1)to ensure that a cyclopentanone product with the purity of more than 99.9% is obtained. Compared with the prior art, the method disclosed by the invention has the advantages that the energy consumption is low; the use efficiency of the catalyst is high; the production cost is greatly reduced; the purity of the obtained cyclopentanone product is more than 99.9%; and a green and environment-friendly production process is provided.
Applications of hydrodeoxygenation catalyst in synthesis of renewable diesel fuel or aviation kerosene
ActiveCN104711012AReduce energy consumptionSimple operation processMetal/metal-oxides/metal-hydroxide catalystsRefining to eliminate hetero atomsAlkaneKerosene
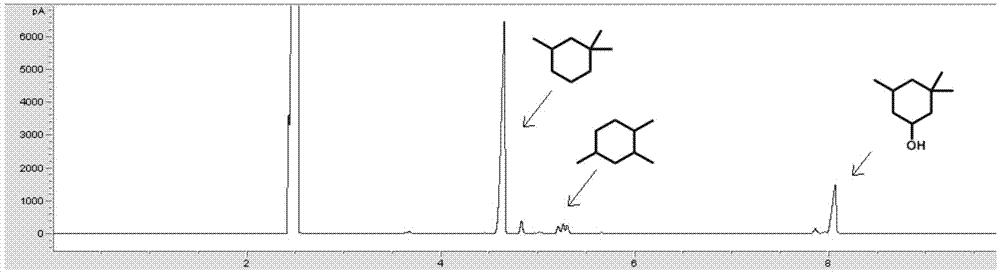
The present invention relates to a new method for preparing diesel fuel or aviation kerosene range hydrocarbons by using isophorone, 3,3,5-trimethyl cyclopentanol, 3,3,5-trimethyl cyclopentanone, 2-ethyl-2-hexenal, 2-ethyl-2-hexanol, 2-ethyl-2-hexanal, fatty acids, fatty acid methyl (or ethyl) esters, a biomass fatty acid triglyceride and other oxygen-containing organic compounds obtained from biomasses as raw materials through a hydrodeoxygenation reaction. According to the present invention, the direct low-temperature hydrodeoxygenation of the biomass oxygen-containing organic compound under the solvent-free condition is achieved, and a series of the high-yield chain alkanes or cycloalkanes having the diesel fuel or aviation kerosene chain length range are obtained; and the catalyst of the present invention has characteristics of no requirement of solvent, simple operation process, mild reaction conditions, good aviation kerosene (or diesel fuel) selectivity, and the like, and is the ideal catalyst for preparation of the diesel fuel or aviation kerosene range hydrocarbon fuels through the hydrodeoxygenation of the biomass oxygen-containing organic compound.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing cyclopentanol through hydration of cyclopentene
ActiveCN102311317AQuick responseReduce dosagePreparation by hydroxy group additionCyclopenteneHydration reaction
The invention discloses a method for preparing cyclopentanol through hydration of cyclopentene. The method comprises a step of continuously performing hydration reaction on the cyclopentene and water through a fixed-bed reactor which holds a strongly acidic cation exchange resin catalyst, wherein the liquid hourly space velocity of the cyclopentene is 0.5h <-1> to 20h <-1>; the molar ratio of the cyclopentene to the water is 0.5-10:1; a reaction solvent is C1-C4 low-carbon alcohol; the amount of the added reaction solvent is 0.05-10 percent of the amount of the cyclopentene; the reaction temperature is 100-200 DEG C; and the reaction pressure is 1.0-6.0MPa. Compared with the prior art, the method has the advantages of single material in a reaction system, high conversion efficiency, high selectivity, fewer byproducts, high convenience in product separation, low energy consumption and the like.
Owner:CHINA PETROLEUM & CHEM CORP +1
Process for preparing cyclopentanol with cyclopentene
InactiveCN102617290AImprove conversion rateHigh selectivityPreparation by alcoholysisCyclopenteneHydration reaction
The invention relates to a process for preparing cyclopentanol with cyclopentene. The process includes the following steps: (1) mixing the cyclopentene with acetic acid, then subjecting the liquid phase to esterification through a catalyst bed formed by sulfonic group cation exchange resins so that the the cyclopentene and the acetic acid can be converted into cyclopentyl acetic ester with the molar ratio of the acetic acid and the cyclopentene being (2-5):1, the space velocity being from 1 to 3 hr-1, the reaction temperature being between 50 DEG C and 80 DEG C, and the reaction pressure being from 0.1 to 0.5 MPa; (2) subjecting the reaction liquid to rectification in a fractionating column with a theoretical plate being 25, recycling the unreacted cyclopentene and acetic acid from the top of the fractionating column, and directly using crude cyclopentyl acetic ester obtained from a column reactor as ester-exchange raw materials; (3) mixing the crude cyclopentyl acetic ester with methyl alcohol, subjecting the mixture to transesterification through a catalyst bed formed by granular calcium oxide (CaO) and finally obtaining the cyclopentanol. Compared with processes in prior art, the process for preparing the cyclopentanol with the cyclopentene enables the reaction conversion ratio and selectivity to be improved obviously, the process is obviously simplified, and the defects of the equipment corrosion and the environment pollution which are caused by using sulfuric acid in the indirect hydration processing of the cyclopentane are overcome.
Method for preparing cyclopentanol from cyclopentene
InactiveCN102603486AImprove efficiencySimple processPreparation by ester-hydroxy reactionPreparation by alcoholysisCyclopentenePtru catalyst
The invention relates to a method for preparing cyclopentanol from cyclopentene, which comprises the following steps: (1) mixing cyclopentene and acetic acid to form a liquid phase, and carrying out an esterification reaction of the liquid phase through a catalyst bed composed of sulfonic acid group cation exchange resin, so that cyclopentene and acetic acid are converted into cyclopentyl acetate, wherein a molar ratio of acetic acid to cyclopentene is (2-5):1, a mass space velocity is (1-3)hr<-1>, a reaction temperature is 50-80 DEG C, and a reaction pressure is 0.1-0.5MPa; (2) carrying out rectification of reaction liquid in a rectification column with the number of theoretical plates of 25, recycling unreacted cyclopentene and acetic acid from the top of the rectification column and cycling and using, and directly using crude acetic acid cyclopentyl ester obtained from a column bottom as a raw material of transesterification; (3) carrying out dehydration of methanol by a 3A molecular sieve, so that the water content of methanol is decreased to below 10ppm; and (4) mixing crude cyclopentyl acetate and methanol, and preparing cyclopentanol through the transesterification, wherein a mass ratio of methanol to acetic acid cyclopentyl ester is (0.8-2.0):1, and the feeding amount of a CaO catalyst is 1-3% of the weight of cyclopentyl acetate. Compared with the prior art, the method provided by the invention has the advantages that the energy consumption is low, the use efficiency of the catalyst is high, and the production cost is greatly reduced.
Method for preparing cyclopentanol from cyclopentene
ActiveCN105461515AImprove conversion rateAvoid difficultiesOrganic compound preparationCarboxylic acid esters preparationCyclopenteneSodium methoxide
The invention relates to a method for preparing cyclopentanol from cyclopentene. Cyclopentene and acetic acid are mixed for an esterification reaction, cyclopentyl acetate is generated, a catalyst adopts cerium nitrate modified sulfonyl cation exchange resin during a transesterification reaction, and the exchange capacity of cerium ions is 10%-30% of that of resin mass; the conversion rate of the esterification reaction is remarkably increased; during the transesterification reaction of crude cyclopentyl acetate and methanol, the transesterification reaction is catalyzed through granular CaO and a sodium methylate composite catalyst dissolved in a reaction liquid, cyclopentyl acetate and methanol can be catalyzed for the transesterification reaction, a small amount of water brought into reaction raw materials can be removed, difficulty caused by CaO hydrolysis and product separation is avoided, and the pollution problem of an existing process is effectively solved.
Owner:SHANGHAI PEARLK CHEM
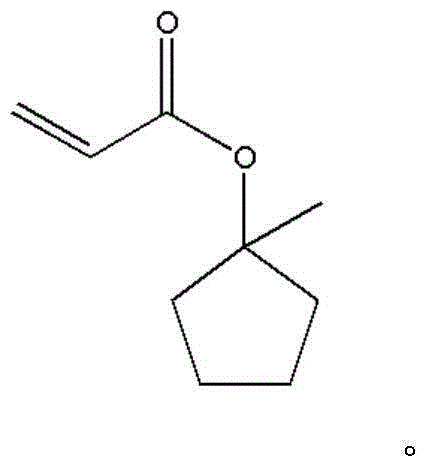
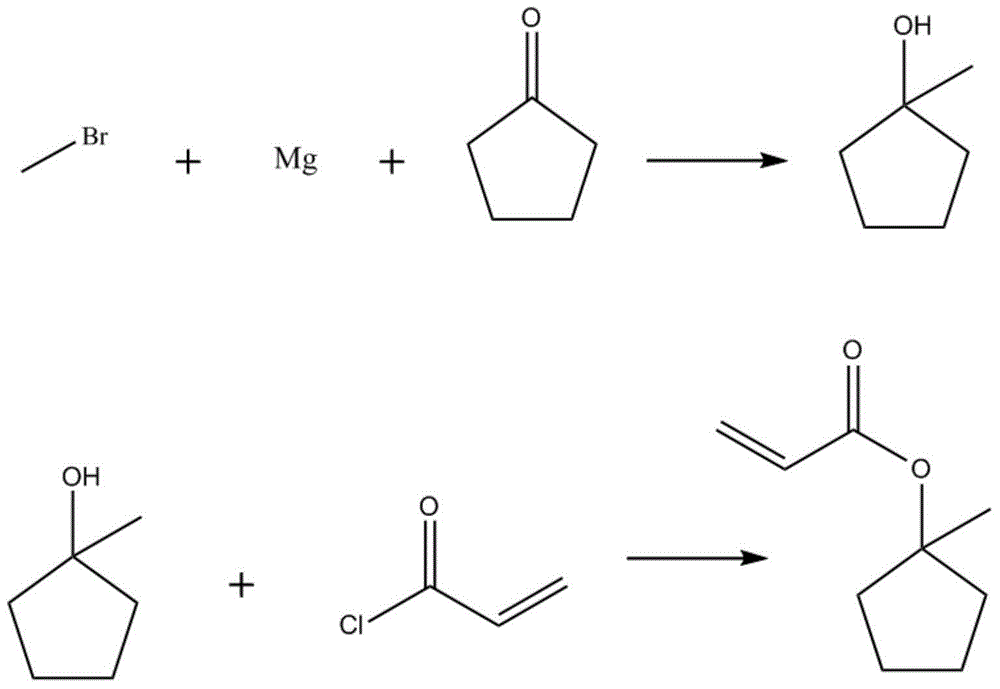
1-methyl-cyclopentanol-acrylate and preparation method thereof
InactiveCN104447311AImprove stabilityWill not aggregatePreparation from carboxylic acid halidesGrignard reagentSynthesis methods
The invention provides a compound 1-methyl-cyclopentanol-acrylate. The compound 1-methyl-cyclopentanol-acrylate is characterized by being shown in a structural formula in the specification. The preparation method comprises the following steps: reacting a grignard reagent with cyclopentanone to generate 1-methyl cyclopentanol, and then reacting with acryloyl chloride, so as to generate 1-methyl-cyclopentanol-acrylate. Through providing a compound and a synthetic route thereof, and optimizing and screening the synthesis method, the problems of low productive rate, complicated reaction, high purification difficulty and the like are overcome; and the product which is high in productive rate, simple in processing process and high in purity is obtained.
Owner:上海博康精细化工有限公司
Anticorrosive self-cleaning paint
InactiveCN104479550ANo shrinkageNo exothermAntifouling/underwater paintsPaints with biocidesDisiloxanePtru catalyst
The invention relates to a self-cleaning paint, particularly an anticorrosive self-cleaning paint. The anticorrosive self-cleaning paint is prepared from the following raw materials in parts by weight: 20-30 parts of tetramethyldivinyl disiloxane, 25-40 parts of methylethoxy silicon oil, 10-15 parts of hydroxy hydrogenous silicon oil, 15-20 parts of chlorotoluene, 1-5 parts of ethanedioic acid, 0.1-0.5 part of cyclopentanol and 0.3-0.7 part of catalyst. The anticorrosive self-cleaning paint can not generate shrinkage or release heat, and has the advantages of moderate hardness, favorable thermal conductivity, favorable electric insulativity and high sealability.
Owner:JIANGSU NUOFEI NEW MATERIAL TECH
Method for preparing cyclopentanone from cyclopentanol by catalytic rectification process
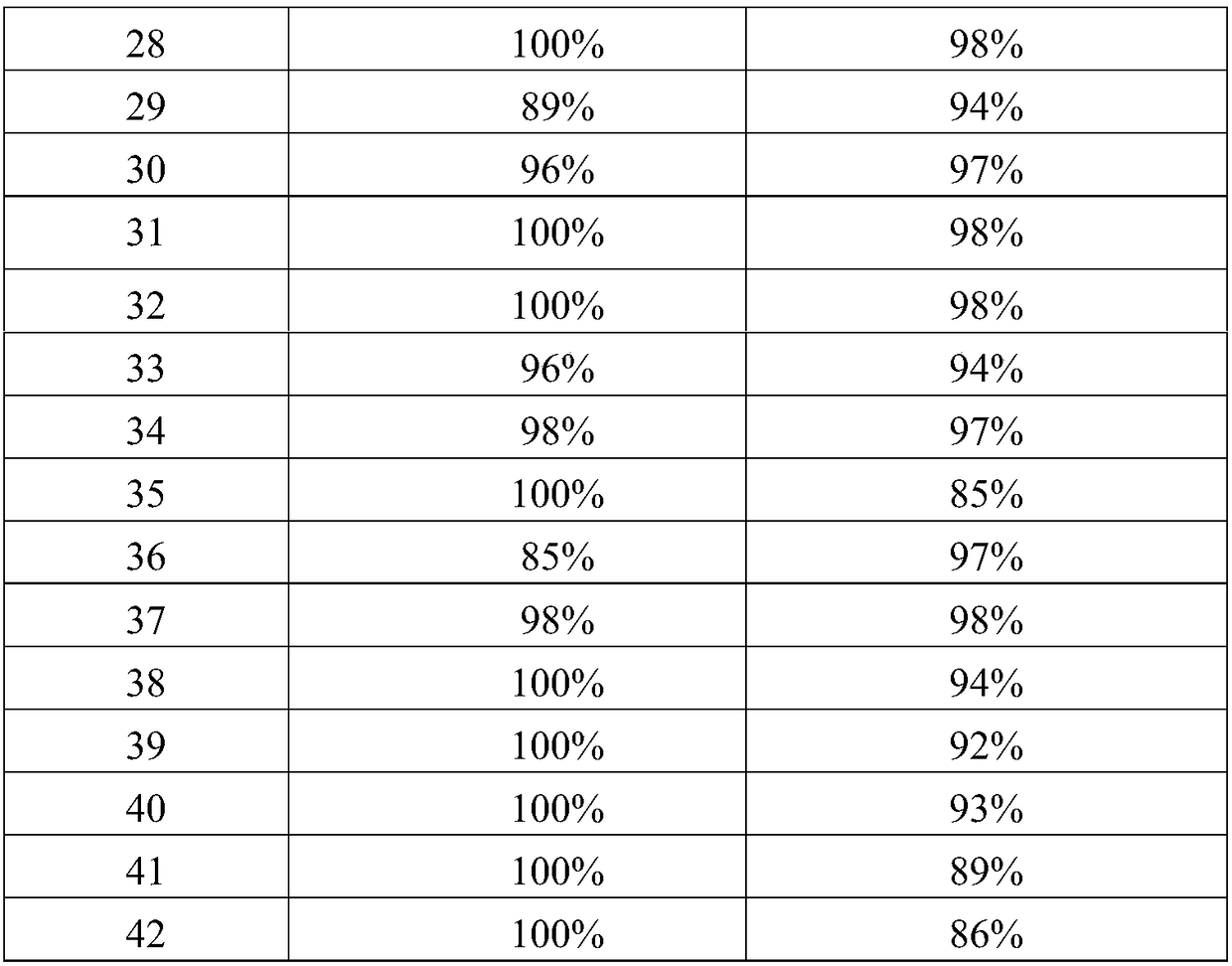
The invention is a kind of method to make the cyclopentanone from the cyclopentanol through the catalysis rectification. We get the cyclopentanone with high purity from the material of cyclopentanol after the catalysis dehydrogenation and rectification reaction. The temperature of the dehydrogenation reaction is between 130deg.C and 140deg.C and at the normal pressure. The catalyzer of the dehydrogenation reaction is granular Raney nickel metal alloy and is composed of Al-Ni-A. The A is one of the Cr, W, Mo or Fe and the weight ratio of the components is Al:Ni:A=1:(0.8 to 0.94):(0.03 to 0.2). The cyclopentanol charge of the catalyzer is between 0.3 hr-1 and 1.5 hr-1 and. The outcome of the dehydrogenation reaction is discharged in the gas phase and can be rectified directly and the reflux ratio is controlled between 1:1 and 10:1. The selectivity of the outcome reaches 100% and the conversion of the cyclopentanol and the purity of the outcome are 98%. It is featured by the low energy consumption, high conversion of the raw material, no by-product, low production cost and green technique.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Method for preparing cyclopentanol through hydratation of cyclopentene
ActiveCN102311316AAvoid separationImprove conversion ratePreparation by hydroxy group additionCyclopenteneWater insoluble
The invention discloses a method for preparing cyclopentanol through hydratation of cyclopentene. The method comprises the following steps of: continuously mixing the cyclopentene and water uniformly by using a static mixer; performing hydratation in a reactor loaded with a strong acid cation exchange resin catalyst; allowing materials obtained through reaction to enter a phase separator for gas-liquid separation; returning unreacted gas phase cyclopentene to the static mixer, and ensuring that the obtained liquid phase contains cyclopentanol and water; and separating products to obtain the cyclopentanol. Compared with the prior art, the method has the advantages that: a reaction system is not added with any solvent or aid, so the technological process is simple, the per pass conversion of the cyclopentene is high, the selectivity of the cyclopentanol is high, a few byproducts are generated, the products are more easy to separate, energy consumption is low, and the method can be applied to the hydratation for water-insoluble C2-C5 hydrocarbons.
Owner:CHINA PETROLEUM & CHEM CORP +1
Automobile exhaust scavenging agent
InactiveCN102941015AEmission reductionLow costDispersed particle separationAmyl alcoholHazardous substance
The invention discloses an automobile exhaust scavenging agent. The scavenging agent comprises materials of, by mass, 10%-20% of butyl alcohol, 5%-10% of amyl alcohol, 15%-30% of cyclopentanol, 5%-15% of benzhydrol, 2%-5% of smoke abatement cleaning agents and 5%-10% of mineral type lubricating agents. According to the automobile exhaust scavenging agent, discharging of harmful substances in engine waste gases can be greatly reduced, the used materials are easy to obtain, and costs are low, so that the automobile exhaust scavenging agent is wide in usage prospect.
Owner:CHANGZHOU UNIV
Method for preparing cyclopentanone through dehydrogenation of cyclopentanol
ActiveCN105461526ACarbonyl compound preparation by oxidationMetal/metal-oxides/metal-hydroxide catalystsReaction temperatureDehydrogenation
The invention relates to a method for preparing cyclopentanone through dehydrogenation of cyclopentanol. According to the method, the cyclopentanone is prepared through dehydrogenation of the cyclopentanol by the aid of a fixed bed layer comprising a Ni-Cu / Al2O3-SiO2 catalyst. The hourly space velocity of a volume liquid for a dehydrogenation reaction is 0.5-2.0 hr<-1>, the system pressure is 0.05-1.0 bar, and the reaction temperature is 100-140 DEG C; the content of an active ingredient, namely, nickel, of the dehydrogenation catalyst Ni-Cu / Al2O3-SiO2 is 30wt%-50wt% of the mass of a supporter, the content of copper of a promoter is 1wt%-5wt% of the mass of the supporter, and the supporter is a mixture of Al2O3 and SiO2; the conversion per pass of the dehydrogenation reaction is higher than 70%, and the selectivity of the cyclopentanone is close to 100%. The reaction efficiency is obviously improved, and the energy consumption is substantially reduced.
Owner:SHANGHAI PEARLK CHEM
Method for preparing cyclopentanol from cyclopentene through hydration
InactiveCN102010296AGood miscibilityNo emissionsPreparation by hydroxy group additionCyclopenteneCyclopentanol
The invention discloses a method for preparing cyclopentanol from cyclopentene through hydration, which comprises the following steps of: adding water and cyclopentene into a high-pressure container in a molar ratio of 4-6:1; adding straight-chain primary alcohol; and under the catalysis of acid cation exchange resin, displacing air in a system twice to four times by using nitrogen, then raising the pressure to 1.0 to 3.0MPa, and reacting at the temperature of between 120 and 160 DEG C for 0.5 to 8 hours to obtain cyclopentanol. In the method, straight-chain primary alcohol with atomic numberof 4 to 6 is used as a reaction solvent; the primary alcohol solvent is introduced into a reaction system, so that not only intersolubility between cyclopentene and water molecules is increased, but also the thickness of a water molecule liquid film formed on the surface of a catalyst and the resistance that cyclopentene is diffused toward the catalyst are reduced. The method is simple and has high reaction efficiency and greatly reduces the energy consumption required by refining. A water phase can be recycled and waste is not discharged almost in the whole process.
Owner:TIANJIN UNIV
Method for preparing cyclopentanol
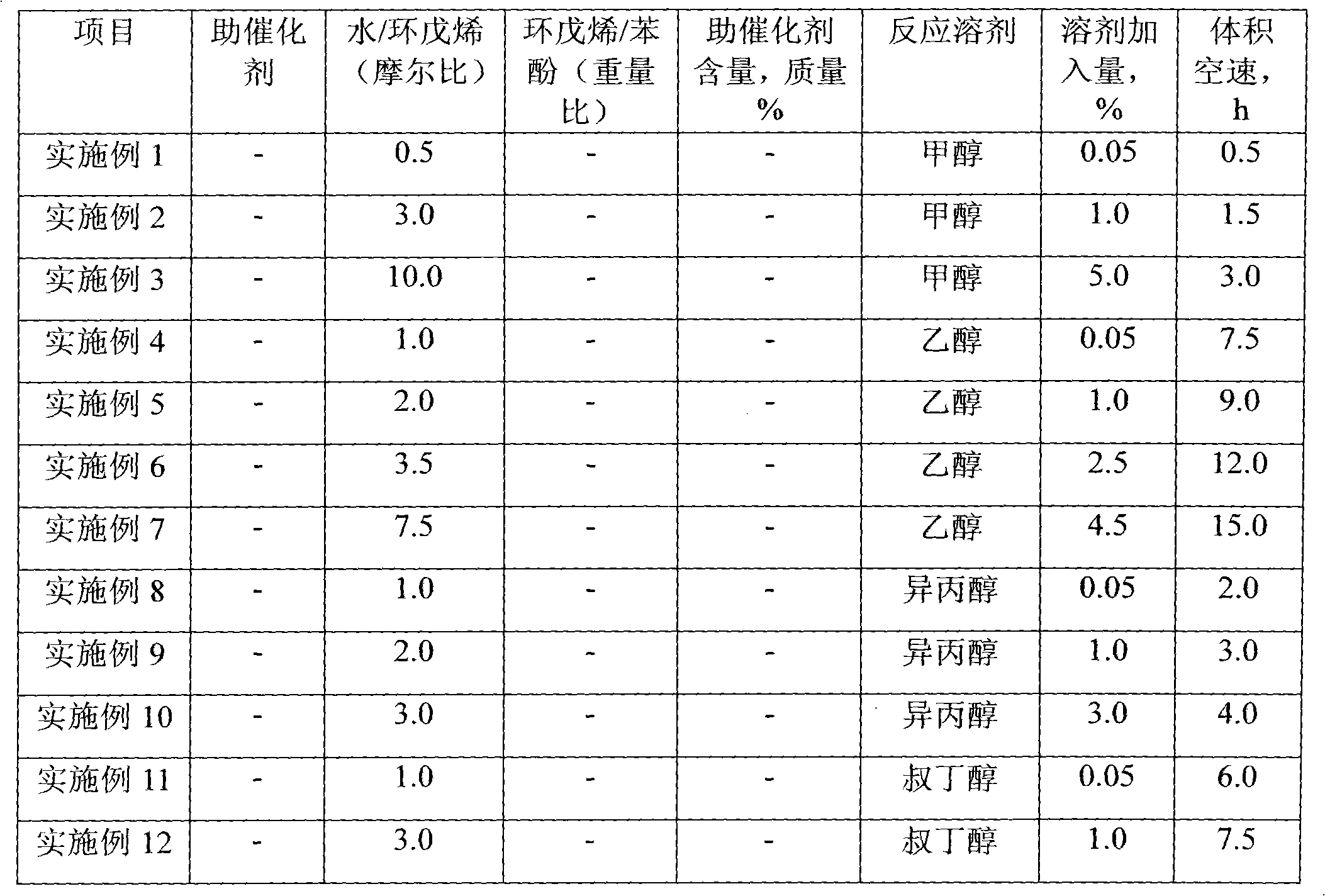
InactiveCN105523893AImprove conversion rateChange surface propertiesPreparation by hydroxy group additionCyclopenteneHydration reaction
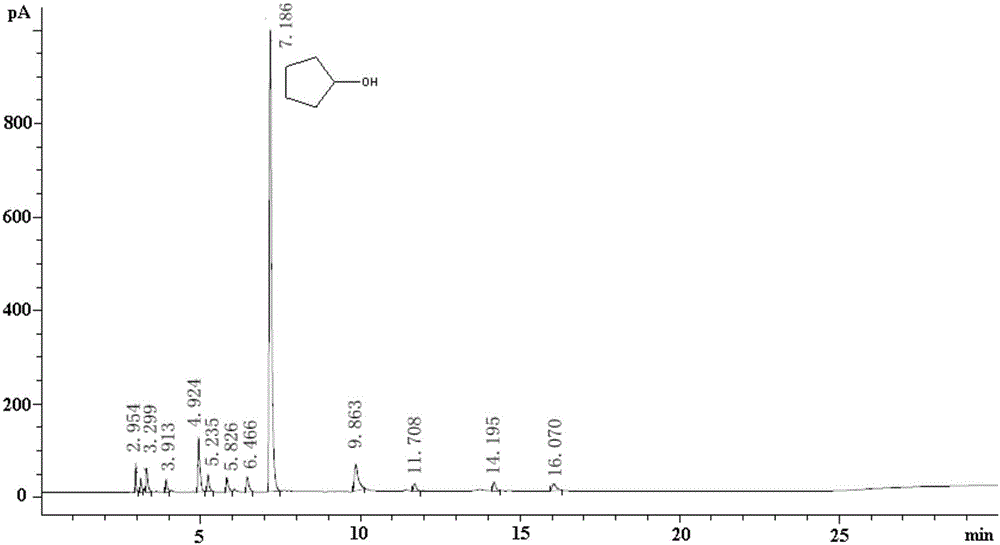
The invention belongs to the technical field of organic chemical industry, and concretely relates to a method for preparing cyclopentanol. According to the method, a material composed of cyclopentene, water and a mixed solvent is continuously performed with a hydration reaction through a fixed bed filled with a catalyst, wherein, the mixed solvent is composed of o-nitrophenol and ketone with weight ratio being (0.5-1.5):1, after the hydration reaction is finished, a reactant is cooled and delaminated to an oil phase and an water phase, the oil phase material is rectificated under normal pressure, fraction at the temperature of 38-55 DEG C is condensed to obtain the unreacted cyclopentene, and the fraction at the temperature of 135-165 DEG C is condensed and collected to obtain refined cyclopentanol. The method has the advantage of high conversion rate for cyclopentene, a solvent is more stable, a side reaction is not generated by cyclopentene and the solvent during a cyclopentanol preparation process through hydration to obtain by-product, refining of cyclopentanol is simpler, and energy consumption is saved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method for cyclopentanol and cyclopentanone by oxidation of cyclopentane
ActiveCN105237355ASimple processMild reaction conditionsPreparation by oxidation reactionsOrganic compound preparationRetention timeDistillation
The invention discloses a preparation method for cyclopentanol and cyclopentanone by oxidation of cyclopentane. The method comprises the following steps: (1) continuously introducing the cyclopentane, a catalyst and oxygen-contained gas into an oxidation reactor and carrying out reaction so as to obtain an oxidation reaction liquid containing the cyclopentanol and the cyclopentanone, wherein the concentration of tail oxygen is controlled within 3% by controlling the introduction amount of the oxygen-contained gas; the usage amount of the catalyst accounts for 1 to 10000 ppm of the weight of the cyclopentane; reaction temperature is 120 to 170 DEG C; reaction pressure is 0.7 to 3.0 MPa; and average retention time of the oxidation reactor in terms of a liquid-phase material is 0.4 to 6 hours; and (2) allowing the oxidation reaction liquid obtained in the step (1) to continuously enter a distillation tower, subjecting a cyclopentane-contained light component obtained from the top of the tower to recycling back to the oxidation reactor, and subjecting crude cyclopentanol and crude cyclopentanone obtained from the bottom of the tower to separation and purification so as to respectively obtain the cyclopentanol and the cyclopentanone. The preparation method provided by the invention has the advantages of mild reaction conditions, high product yield, good selectivity, greenness and environmental protection.
Owner:山东友道化学有限公司
Method for preparing cyclopentanol
ActiveCN108069819AHigh activityImprove stabilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidCyclopentene
The invention discloses a method for preparing cyclopentanol. The method comprises steps as follows: acetic acid and cyclopentene are taken as reaction materials and subjected to an addition reactionin a fixed bed continuous reaction device filled with an ion exchange resin catalyst, the reacted materials and water enter a catalytic rectifying tower reboiler, azeotropic steam rises to a catalyticrectifying tower reaction section filled with a modified ion exchange resin catalyst for a hydrolysis reaction, little water is added to the top of the reaction section in the reaction process, a hydrolysate is distilled from the tower top and purified, and a cyclopentanol product is obtained. According to the method, the reaction process is simple, the conditions are mild, and the catalyst has stable activity and can operate in a long cycle.
Owner:FUSHUN RES INST OF PETROLEUM & PETROCHEMICALS SINOPEC CORP +1
Method for preparing cyclopentanol or cyclopentanone by catalytically converting biomass
InactiveCN108821941AEasy to prepareReduce manufacturing costOrganic compound preparationPreparation by oxygen reductionAlcoholHydrogen
The invention relates to a method for preparing cyclopentanol or cyclopentanone by catalytically converting biomass. A Cu-containing catalyst is used, deionized water is used as a solvent, furfural orfurfuryl alcohol is added into an autoclave, hydrogen is introduced, and a reaction is carried out a certain reaction temperature to obtain the product cyclopentanol or cyclopentanone. The catalyst has the advantages of simple preparation process, good catalysis effect in the catalysis of highly-selective hydrogenation of furfural or furfuryl alcohol under controlled reaction conditions to prepare the cyclopentanol or cyclopentanone, easiness in recovery and reuse, and excellent industrial application prospect.
Owner:NANJING UNIV OF TECH
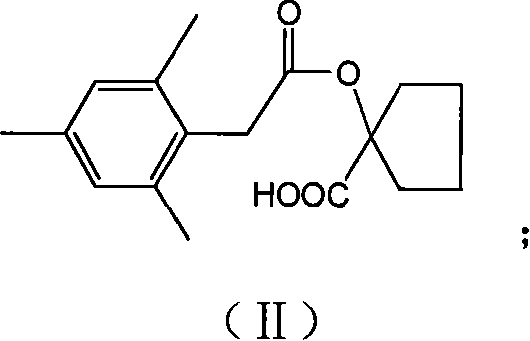
Preparation method of spiromesifen
The invention discloses a preparation method of spiromesifen, which uses 1-carboxyl cyclopentanol, 2, 4, 6-trimethylbenzene dichloroacetyl chloride and 3, 3-dimethyl butyryl chloride as main materials. The preparation method comprises (1), generating compound II: reacting 1-carboxyl cyclopentanol and 2, 4, 6-trimethylbenzene dichloroacetyl chloride in the presence of catalyst and in organic solvent, (2), generating compound III by heating the compound II and strong alkali in alcohol solution or in aprotic strong polar solvent to process reflux reaction, (3), generating spiromesifen by reacting the compound III and 3, 3-dimethyl butyryl chloride in the presence of catalyst and in organic solvent, wherein the prepared spiromesifen is represented as above. The preparation method of spiromesifen is suitable for industrial production.
Owner:ZHEJIANG UNIV
Method for preparing cyclopentanol from cyclopentene
PendingCN110818566AEasy to makeImprove efficiencyOrganic compound preparationCarboxylic acid esters preparationCyclopenteneAcetic acid
The invention discloses a method for preparing cyclopentanol from cyclopentene and acetic acid and co-producing ethanol. The method comprises the following steps: firstly, performing an addition reaction on cyclopentene and acetic acid under the action of an acid catalyst, and distilling and purifying the obtained product so as to obtain cycloamyl acetate; and performing a hydrogenation reaction on the cycloamyl acetate under the action of a metal catalyst, so as to obtain cyclopentanol and ethanol. The reaction process proposed by the invention not only has a high single-pass conversion rateand good reaction stability, but also does not use any solvent, has the characteristics of high efficiency, environmental protection and no pollution, and has a good industrial production prospect.
Owner:中科榆林能源技术运营有限责任公司
Energy-containing combustion-supporting catalyst and application thereof
InactiveCN102585936AImprove thermal efficiencyImprove heat utilizationGaseous fuelsLiquid carbonaceous fuelsBorideAlkane
The invention relates to an energy-containing combustion-supporting catalyst which mainly is composed of the following components by weight: 25-28 parts of fusel oil, 14-18 parts of ethanol, 16-20 parts of tetralin, 14-16 parts of cyclopentanol, 18-22 parts of crotonic aldehyde, 1-2 parts of 2-pyrrolidone, 1.5-3 parts of nano rare earth oxide, and 2-3 parts of energy-containing cage-type boride or derivates of the boride. The energy-containing combustion-supporting catalyst can be applied to liquid or gas alkane fuel to form an energy-containing composite fuel so as to promote the thermal efficiency of the fuel, and can effectively replace acetylene to be used for metal flame welding cutting so as to improve the heat utilization rate of gas boilers and kilns. The energy-containing combustion-supporting catalyst is obvious in energy-saving efficiency, and saves fuel by more than 30 percent and saves oxygen by more than 20 percent in the process of metal flame welding cutting. Furthermore, compared with acetylene, the energy-containing combustion-supporting catalyst is safe in use, and is unlikely to cause tempering, and is safe and reliable in use.
Owner:栗伟鹤
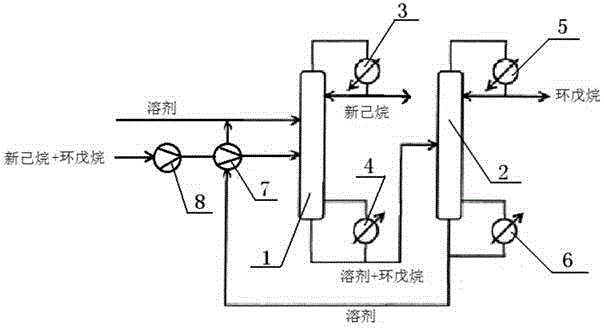
Method for separating cyclopentane and neohexane by using extractive rectification technology
ActiveCN105859506AChange intermolecular forcesEnhanced mass transferDistillation purification/separation1-PentanolN dimethylformamide
The invention discloses a method for separating cyclopentane and neohexane by using an extractive rectification technology, which belongs to the field of petrochemical industry. A solvent used in the extractive rectification technology comprises the following components by mass part: 1.5-9 parts of a main solvent and 1 part of an auxiliary solvent, wherein the main solvent is N,N-dimethylformamide and / or N-formyl morpholine, and the auxiliary solvent is at least one of 1-butanol, 2-butanol, 1-amyl alcohol, 2-amyl alcohol, cyclopentanol, cyclohexanol, ethylene glycol, propylene glycol, methyl acetate, ethyl acetate, and phenol. The provided method has excellent separating effect for cyclopentane and neohexane as well as has advantage of low energy consumption.
Owner:PETROCHINA CO LTD +2
Method for preparing fertilizer based on biomass charcoal
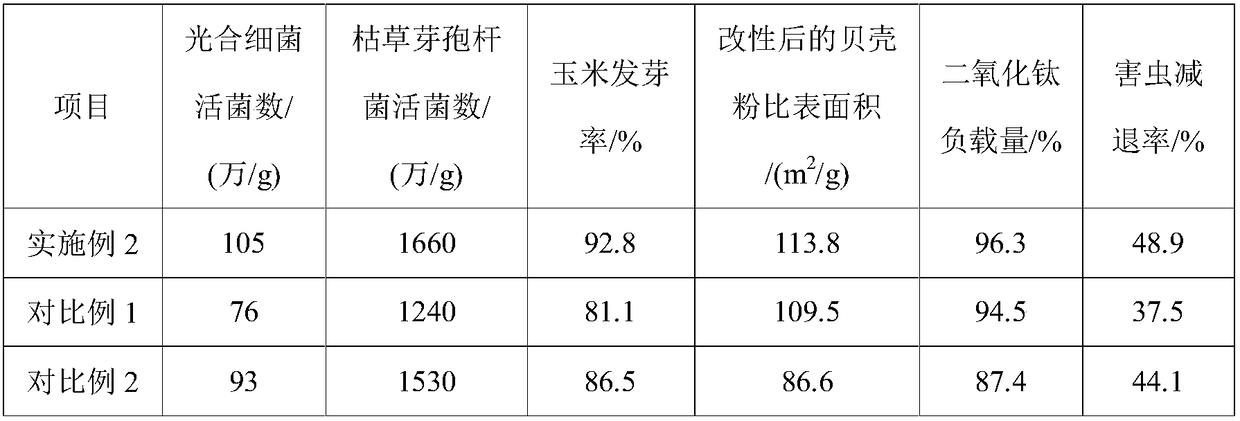
InactiveCN109206214ASimple ingredientsIngredients easy to getAnimal corpse fertilisersAlkali orthophosphate fertiliserDiseasePrill
The invention provides a method for preparing a fertilizer based on biomass charcoal, belonging to the field of fertilizers. According to the invention, modified shell powder and other raw materials are combined and mixed to prepare a bio-organic-inorganic compound fertilizer; the shell powder has a large specific surface area and forms a colloidal film on the surface of a fertilizer particle, andthe shell powder, together with amino acids and beneficial trace elements rich in the shell powder, constitutes a beneficial microbial culture medium for soil on the surface of the fertilizer particle to promote the mass propagation of microorganisms; at the same time, antibacterial and bactericidal abilities are obtained through loading of active nanometer titanium dioxide; and the modificationof the shell powder is catalyzed through calcination of a mixed reagent of titanium dioxide, sodium citrate and 1-(3-hydroxy-n-propyl)cyclopentanol. The fertilizer prepared in the invention realizes optimal application effect in different time periods through compounding of microbial agents with different proportions and has the advantages of promotion of crop growth, improvement of soil structureand colony groups, resistance to pests and diseases, etc.
Owner:卫卓然
Preparation method of ticagrelor midbody
ActiveCN103275056AStable in natureEasy to addOrganic chemistryBulk chemical productionAlcoholTicagrelor
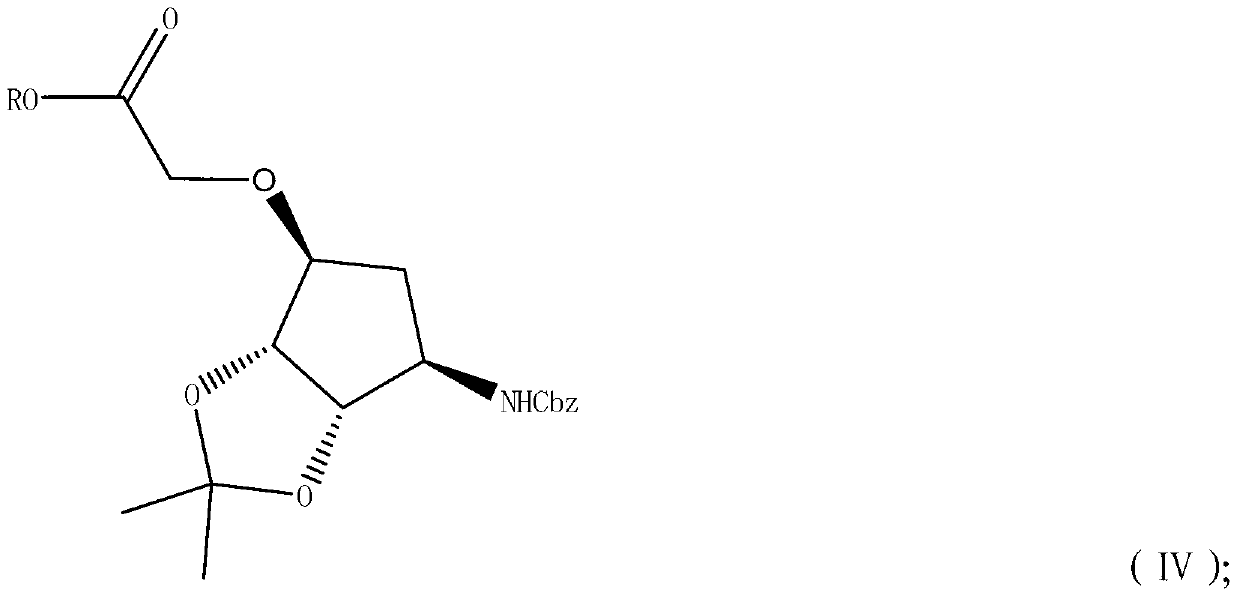
The invention discloses a of a ticagrelor midbody. The preparation method comprises the following steps of: by taking chiral cyclopentanol protected by N-Cbz under an alkaline condition as a starting material, converting the starting material to a compound IV by utilizing an etherification reaction, then reducing under a system of a reducing agent and alcohol to obtain a compound V, and at last, removing protecting groups to obtain a icagrelor midbody I. The whole reaction process is short in time; the purity of the product is high; the yield is high; and each midbody is easily purified. The needed materials are cheap and easily-available, so that the preparation preparation saves time and production cost, is favorable for large-scale industry production and has important significance.
Owner:ZHEJIANG APELOA JIAYUAN PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com