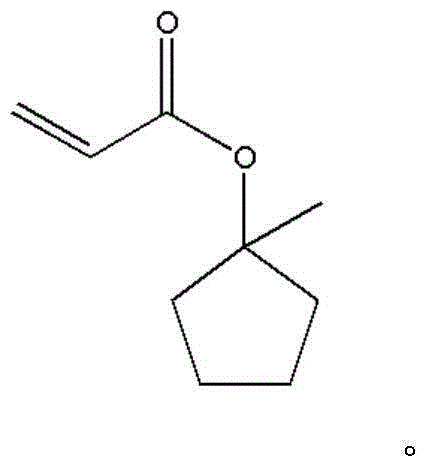
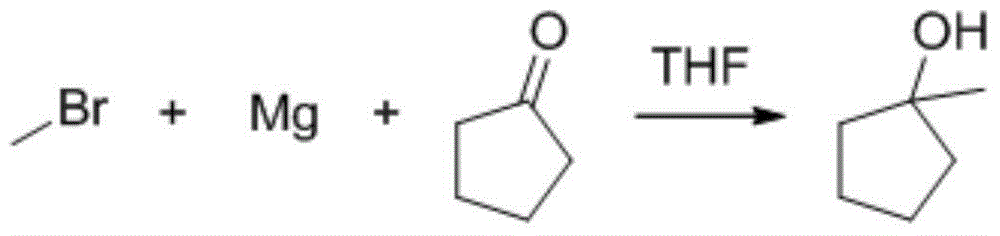1-methyl-cyclopentanol-acrylate and preparation method thereof
A technology of methyl cyclopentanol and acrylate, applied in the preparation of carboxylic acid halide, organic chemistry, etc., can solve the problems of low reaction yield, many side reactions, unsuitable for industrial production, etc., and achieves simple operation process and method. Simple and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1), the reaction equation is as follows:
[0051]
[0052] (2), specific process steps:
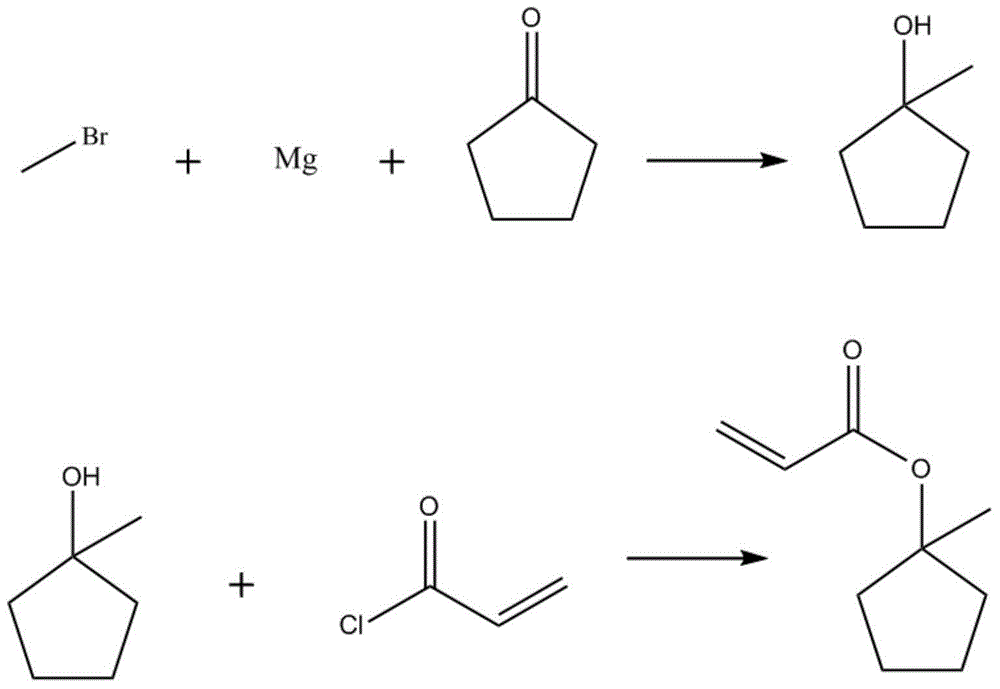
[0053] Into a 10L four-necked reaction flask, 4L of tetrahydrofuran and 482g of magnesium chips were added under nitrogen protection. At room temperature (8°C), 3363g of methyl bromide THF solution with a mass concentration of 61.8% was added dropwise, stirred to initiate, and the temperature was controlled to A , while cooling down naturally (from 65°C to 10°C), the solution becomes very viscous, (center control 1).
[0054]Slowly pour the reaction mixture into 5Kg (or 4kg, 5.5kg, 6kg or 6.5kg) of ice under stirring, then directly pour 1.6L concentrated HCl into the above reaction mixture under stirring, pH4 Dry, filter, and remove THF and DCM by rotary evaporation to obtain 1.9Kg of crude product (in-control 2).
[0055] Water pump vacuum distillation, external temperature 80-90°C, front fraction 41-49°C, 95g, (in-control 3). The external temperature is 90-95°C, the main fr...
Embodiment 2
[0061] (1), the reaction equation is as follows:
[0062]
[0063] (2), specific process steps:
[0064] Add 451g of A, 815g of acryloyl chloride, 3L of DCM, 9.0g (or 1.0g, 5.0g, 11.0g, 15g) of phenothiazine into a 10L four-neck flask, and cool to -10°C. 1002 g of triethylamine was added dropwise within 2 hours, solids were precipitated, and the reaction solution turned yellowish brown, and the color continued to deepen. The control temperature is less than 10°C. After the addition, react at room temperature (10° C.) for 1.5 h (in-control 1). Add 3L (or 2L, 3.5L, 4L) of saturated NaCl solution, separate the layers, extract the aqueous phase with (1.2L×3 times) DCM, combine the organic phases, and wash with (2L×2 times) saturated NaCl solution, without Dry over sodium sulfate, filter, and remove the solvent by rotary evaporation.
[0065] The crude products of the two batches of reactions were combined to a total of 2.4Kg, 2g (or 1.0g, 1.2, 0.7g, 0.1g) of p-tert-butylcat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com