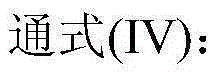Preparation method for cyclopentanol and cyclopentanone by oxidation of cyclopentane
A technology for cyclopentanol and cyclopentane, which is applied in the field of preparing cyclopentanol and cyclopentanone, can solve the problems of large circulation of cyclopentene as a raw material, difficulties in the separation and purification process, and difficulties in the recovery of concentrated sulfuric acid, and achieve low cost and reduced The effect of reducing production cost and separation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
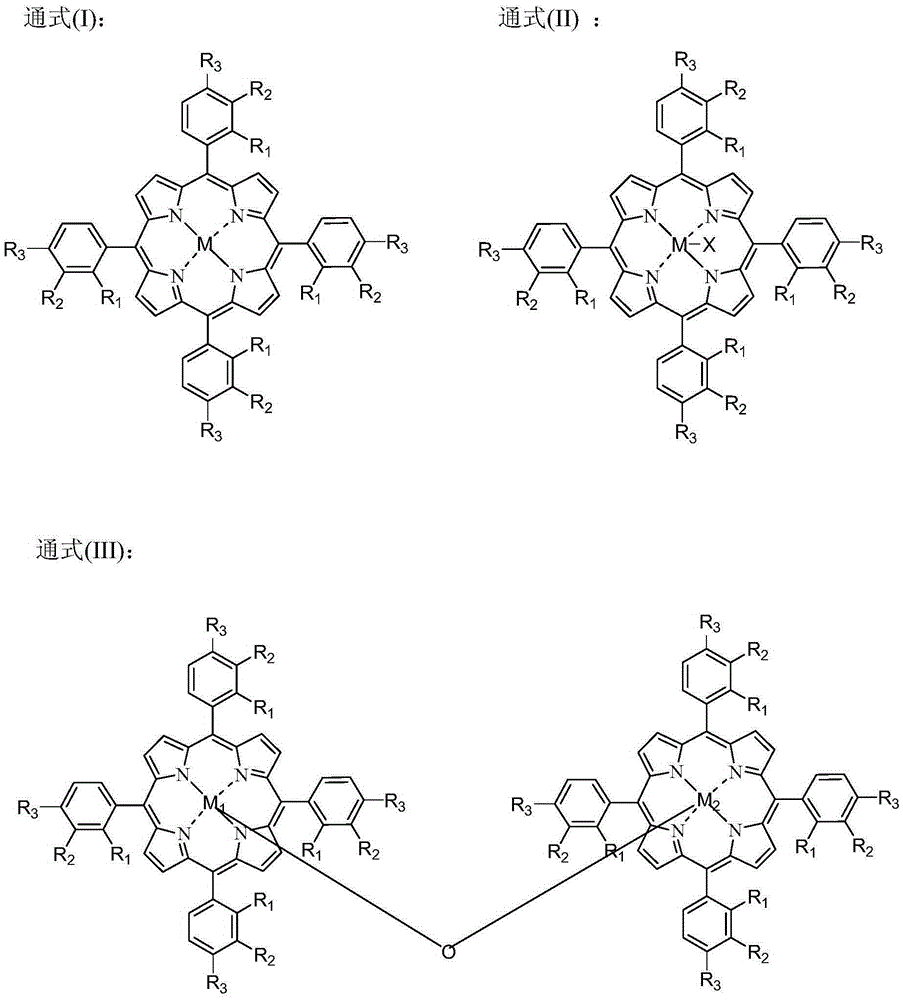
[0049] The catalyst dissolved in fresh cyclopentane added to the system is Co(Ac) 2 .4H 2 O, metal phthalocyanine (R) with general formula (IV) structure 1 =CH 3 CH 2 , R 2 =H, M=Mn), the metalloporphyrin (R 1 =R 2 = H, R 3 =CH 3 , M=Cu) mixture with a total concentration of 225 ppm. When the device is in steady state operation, the flow rate of fresh cyclopentane added to the oxidation reactor is 15.5mL / h. At this time, the average residence time of the oxidation reactor in terms of liquid phase substances is 0.4h, and the pressurized air is continuously fed into the In the oxidation reactor, the reaction temperature of the system is maintained at 165° C., and the reaction pressure is 2.0 MPa. After sampling analysis, the conversion rate of cyclopentane in the system is 99.1%, and the total selectivity of cyclopentanol and cyclopentanone is 98.4%, wherein the selectivity of cyclopentanol is 62.6%, and the selectivity of cyclopentanone is 35.8%. %.
Embodiment 2
[0051] The catalyst that dissolves in the fresh cyclopentane that adds system is N-hydroxyl phthalimide, the metal phthalocyanine (R with general formula (IV) structure 1 =OH,R 2 =H, M=Ru) and metalloporphyrins (R 1 =R 3 = H, R 2 =OH,M 1 = M 2 =Mn), the total concentration is 10000ppm. When the device is in steady state operation, the flow rate of fresh cyclopentane added to the oxidation reactor is 1.2mL / h. At this time, the average residence time of the oxidation reactor in terms of liquid phase substances is 6.0h, and the pressurized pure oxygen is continuously passed through into the oxidation reactor to maintain the system reaction temperature at 120°C and the reaction pressure at 0.7MPa. After sampling analysis, the conversion rate of cyclopentane in the system is 99.4%, and the total selectivity of cyclopentanol and cyclopentanone is 98.0%, wherein the selectivity of cyclopentanol is 58.4%, and the selectivity of cyclopentanone is 39.6%. %.
Embodiment 3
[0053] The catalyst dissolved in the fresh cyclopentane added to the system is Cr(NO 3 ) 4 , N-acetyl phthalimide, metal phthalocyanine (R 1 =OCH 3 , R 2 =H, M=Ni) and the metalloporphyrin (R 1 =R 3 =OCH 3 , R 2 =H, M=Fe) with a total concentration of 7800 ppm. During the steady state operation of the device, the flow rate of the fresh cyclopentane added in the oxidation reactor was 2.2mL / h, and the average residence time of the oxidation reactor in terms of liquid phase material was 3.5h at this moment, and the oxygen concentration was 76% The pressurized oxygen enrichment is continuously passed into the oxidation reactor, and the reaction temperature of the system is maintained at 132° C., and the reaction pressure is 0.9 MPa. After sampling analysis, the conversion rate of cyclopentane in the system is 99.6%, and the total selectivity of cyclopentanol and cyclopentanone is 97.8%, wherein the selectivity of cyclopentanol is 56.7%, and the selectivity of cyclopentanon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com