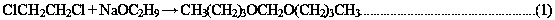Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Amberlyst-15" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Search results for amberlyst 15 at Sigma-Aldrich. Compare Products: Select up to 4 products. *Please select more than one item to compare
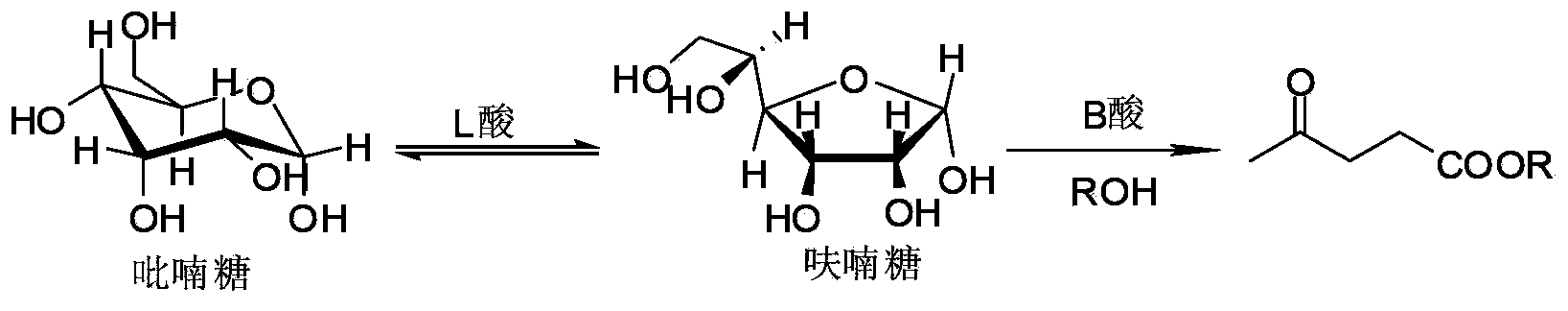
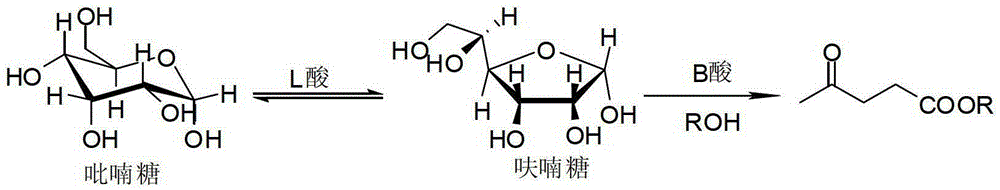
High-yield levulinic acid ester preparation method under catalysis of solid acid
ActiveCN103408422AHigh yieldCatalytic conversionOrganic compound preparationCarboxylic acid esters preparationFood additivePropanoic acid
The invention discloses a high-yield levulinic acid ester preparation method under catalysis of solid acid and belongs to the field of organic synthesize. The method comprises the following steps: uniformly mixing 1 part of sugar, 10-100 parts of alcohol, 0.01-0.2 part of acid B and 0.1-1 part of acid L, and performing reaction under the temperature of 130-190 DEG C for 1-20 h to obtain a levulinic acid methyl ester solution, wherein the acid L is stanniferous molecular sieve, and the acid B is SBA-15-SO3H, Carbon-SO3H or Amberlyst-15. According to the method, the carbohydrate is low in cost and easy to obtain; with the acid B and acid L which can be used repeatedly, the catalysis effect basically remains the same; the levulinic acid ester compound prepared in one step can be widely applied to industries of food additives, essence spices, chemical reaction intermediates, gasoline, diesel-dopes and the like.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
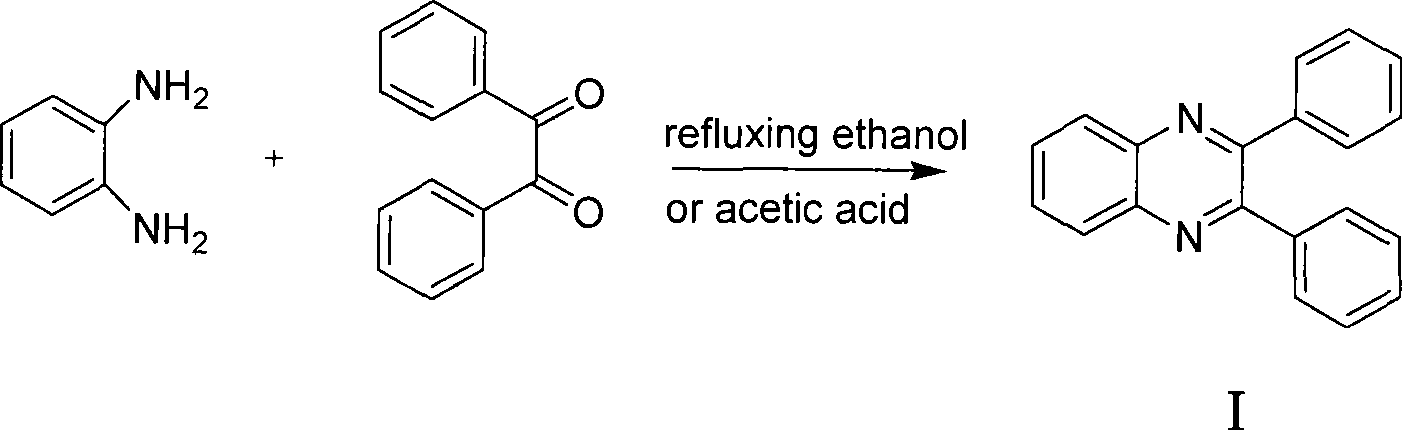
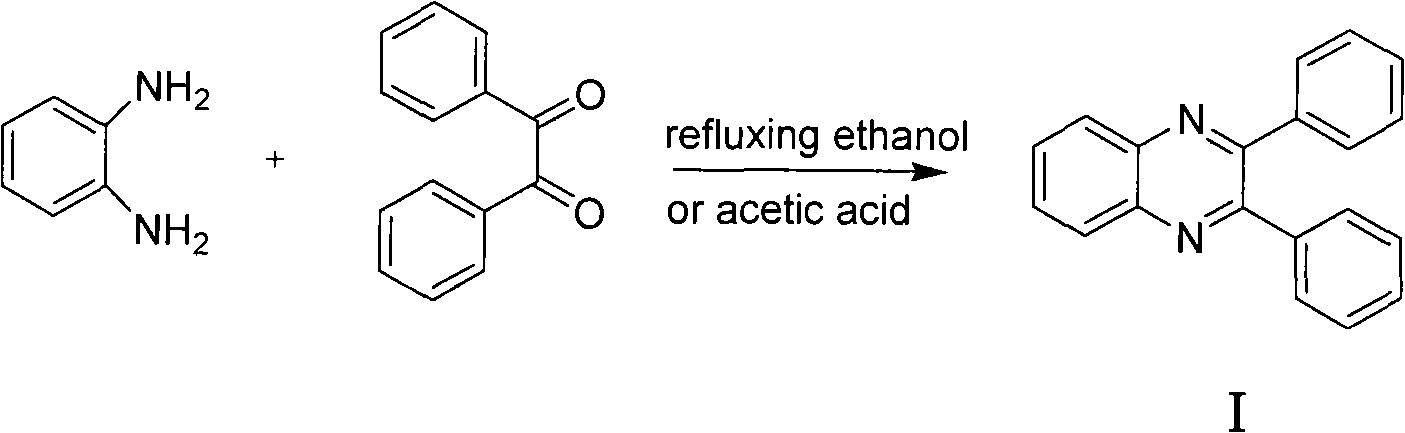
Preparation of quinoxaline derivatives
InactiveCN101481357ANot easy to cause pollutionShorten the timeAntibacterial agentsOrganic chemistryQuinoxalineOrganic solvent
The invention discloses a preparation method of a quinoxaline derivative. The method is characterized by condensing an o-diamino compound with an o-dicarbonyl compound in a polar solvent in the presence of catalyst Amberlyst-15 or NKC-9 dry hydrogen catalyzed resin Styrene-DVB to prepare the quinoxaline derivative. The preparation method takes ordinary tap water instead of a toxic organic solvent as the solvent for a condensation reaction to obtain the quinoxaline derivative with high yield and high content. The catalyst can be recovered and can be reused for at least three times in a condition of no loss of the catalytic activity, thus effectively reducing the production cost. The preparation method of the quinoxaline derivative has the advantages of simple process operation, no environment pollution, and is fit for large-scale industrialized production.
Owner:TIANJIN NORMAL UNIVERSITY
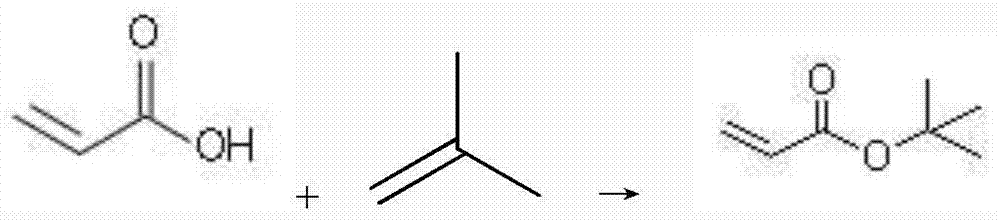
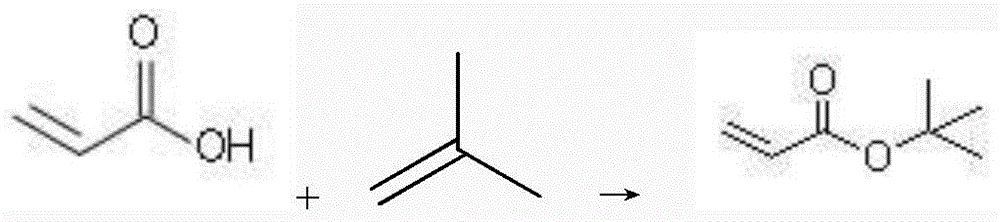
Method used for catalyzed synthesis of tert-butyl acrylate
InactiveCN104844455ASimple stepsHigh yieldOrganic compound preparationCarboxylic acid esters preparationFiltrationAmberlyst-15
The invention relates to a method used for catalyzed synthesis of tert-butyl acrylate. The method comprises following steps successively:1) acrylic acid, a catalyst combination, a polymerization inhibitor A, and a polymerization inhibitor B are delivered into a sealed reaction kettle, and liquefied isobutylene is added dropwise for esterification, wherein the catalyst combination is composed of nanosolid superacid SO<4><2-> / SnO<2>, Amberlyst-15, D001 resin, and NKC-9 resin; 2) after adding, heat preservation is carried out; 3) after heat preservation, the sealed reaction kettle is subjected to pressure relief, an obtained mixture is subjected to filtration, and an obtain liquid ingredient is pumped into a rectifying tower; and 4) by-products tert-butyl alcohol, diisobutylene, and the target product tert-butyl acrylate are obtained via separation successively. Yield of tert-butyl acrylate is higher than 98%; reuse ratio of the catalyst is high; the method is simple; and no pollution is caused.
Owner:徐德良 +1
Method for preparing 5-ethoxymethylfurfural by glucose
ActiveCN108658904AEasy to recycleReduce manufacturing costOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsIon exchangeAmberlyst-15
The invention discloses a method for preparing 5-ethoxymethylfurfural by glucose. The method comprises the following steps of using the glucose as a reaction primer, using an anhydrous ethyl alcohol-dimethyl sulfoxide cosolvent system as a reaction medium, and using combination of aluminum trifluoromethanesulfonate and ion exchange resin Amberlyst 15 as a synergistic catalyst, so as to form a conversion reaction system; reacting at the temperature of 140 to 160 DEG C, so as to obtain the 5-ethoxymethylfurfural. The dimethyl sulfoxide is used as a cosolvent, so as to effectively inhibit the generation of side reaction; the glucose is converted into the target product at high selectivity, namely the 5-ethoxymethylfurfural; the maximum yield rate reaches 48.8%. The preparation method has theadvantages that the preparation method is simple and effective, the catalyst is easy to recycle and repeatedly use, the glucose with wide source and low cost is used as the reaction raw material, theeconomic benefit is high, and the good industrial application prospect is realized.
Owner:KUNMING UNIV OF SCI & TECH
Attapulgite-reinforced resin solid acid catalyst and preparation method thereof
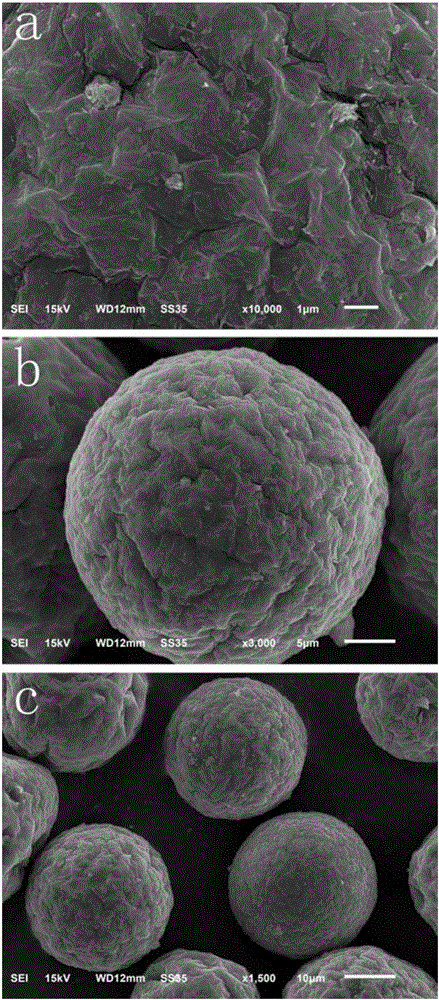
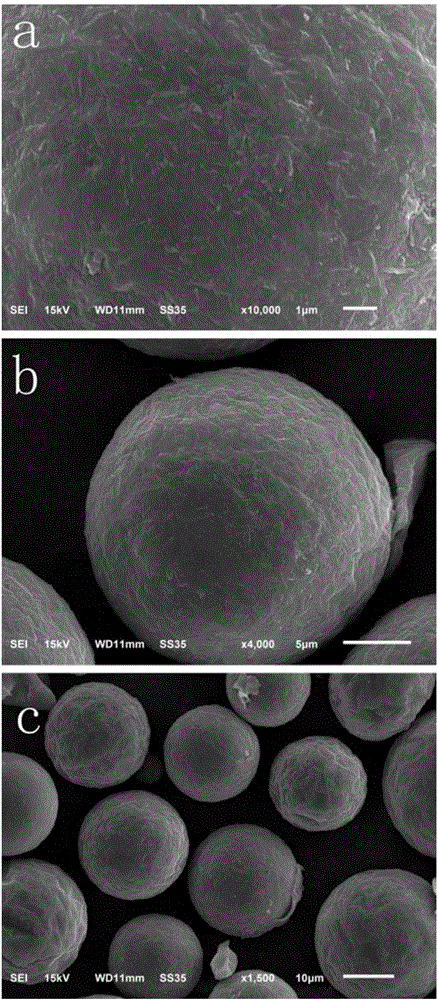
InactiveCN106824283AUnique core-shell structureGood anti-swelling performanceOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSolid acidScanning electron microscope
The invention discloses a novel attapulgite-reinforced resin catalyst and a preparation method thereof. The novel solid acid catalyst is prepared by suspension polymerization by using attapulgite as an independent dispersing agent. The preparation method comprises the following steps: modifying attapulgite by using a silane coupling agent until the surface has appropriate hydrophilicity / hydrophobicity; synthesizing attapulgite / polystyrene microspheres by using the modified attapulgite as a dispersing agent; and finally, sulfonating by concentrated sulfuric acid to obtain the attapulgite-reinforced resin solid acid catalyst. The structure of the catalyst is determined by Fourier-infrared spectrum and a scanning electron microscope. In the lactic acid-butanol reaction, compared with the Amberlyst-15, the catalyst disclosed by the invention has better properties, stable structure and excellent anti-swelling property.
Owner:NANJING UNIV OF TECH
Synthesis method of high-4,4'-isomer-content bisphenol F
InactiveCN105037107ASuitable for continuous productionLow reaction temperatureOrganic chemistryOrganic compound preparationSynthesis methodsReaction temperature
The invention discloses a synthesis method of high-4,4'-isomer-content bisphenol F, which comprises the following step: by using phenol and formaldehyde as raw materials, synthesizing bisphenol F by a two-step process by using Amberlyst-36 and Amberlyst-15 cation exchange resins as catalysts, wherein the mass percent of the 4,4'-isomer bisphenol F is up to 60% or above, and the mass yield of the bisphenol F is up to 90% or above. The catalysts are commercial cation exchange resins, can be easily purchased, separated and recovered, and are recyclable. The method has the advantages of low reaction temperature, short reaction time and no corrosiveness to equipment, is suitable for continuous production of tubular reactors, and has favorable industrial application prospects.
Owner:XIANGTAN UNIV
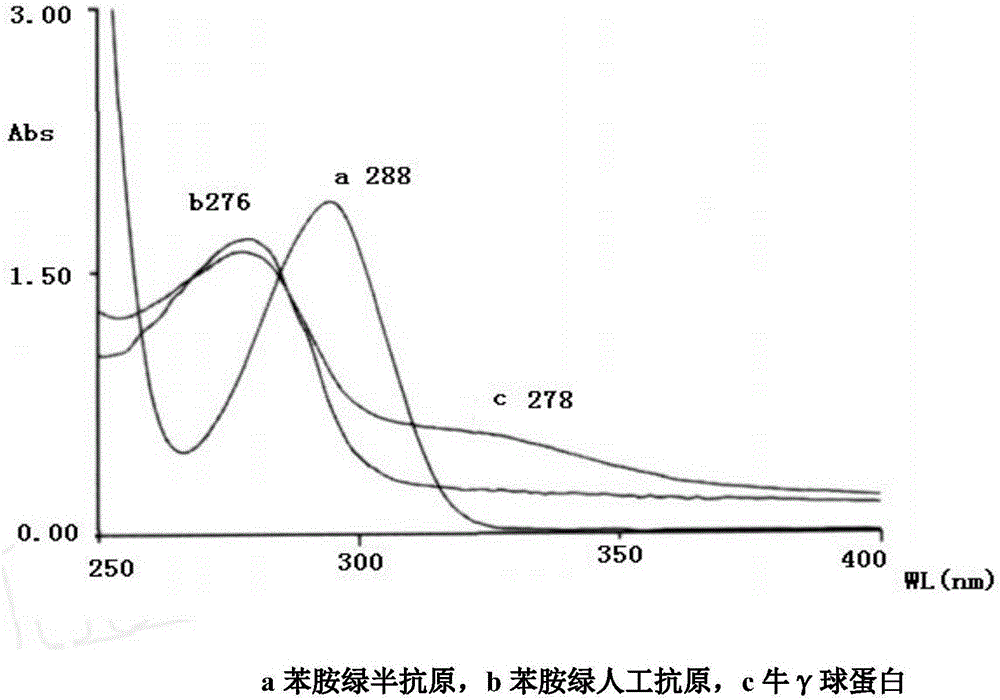
Method for preparing aniline green artificial antigens
InactiveCN106046143AAdvanced synthesis technologyStrong specificityOrganic compound preparationAmino-carboxyl compound preparationDimethylaniline N-oxideAmberlyst-15
The invention provides a method for preparing aniline green artificial antigens. The method includes steps of firstly, preparing and detecting haptens, and to be more specific, synthesizing the aniline green haptens from N, N-dimethylaniline and benzaldehyde derivatives by the aid of Amberlyst 15 resin under certain conditions; secondly, preparing and detecting artificial antigens, to be more specific, respectively coupling the obtained haptens and bovine gamma globulin (BGG) by the aid of active ester and diazotization processes and preparing the aniline green artificial antigens which are corresponding complete antigens formed by aniline green-bovine gamma globulin. The Amberlyst 15 resin is used as a catalyst. The method has the advantages that animal immunization can be carried out on the aniline green artificial antigens to obtain corresponding aniline green antibodies, the method can be used for researching diversified aniline green immunoassay processes, and convenient, quick and accurate ways can be provided for aniline detection.
Owner:HANGZHOU LAIHE BIOTECH CO LTD
Method for directly preparing alkyl levulinate from furfural
ActiveCN108675928ALow costEasy to synthesizeOrganic compound preparationCarboxylic acid esters preparationMolecular sieveHydrogen
The invention discloses a method for directly preparing alkyl levulinate from furfural. The method is characterized in that the furfural is used as raw materials; low-grade alkanol is used as a reaction medium; a combination of a supported molecular sieve ZrO2 / MCM and ion exchange resin Amberlyst-15 is used as a catalyst; reaction is performed for 8 to 24h at 130 to 150 DEG C; an alkyl levulinateproduct with the yield reaching up to 85.3 percent can be obtained. In the process, the used catalyst system is cheap and can be easily obtained; the catalyst can be easily separated, recovered and repeatedly used; the one-step synthetic process is simple; the equipment is simplified; the reaction conditions are mild; the additional addition of hydrogen gas is not needed; the safe and environment-friendly effects are achieved; the conversion efficiency is high; the target product yield is high; good industrial application prospects are realized.
Owner:KUNMING UNIV OF SCI & TECH
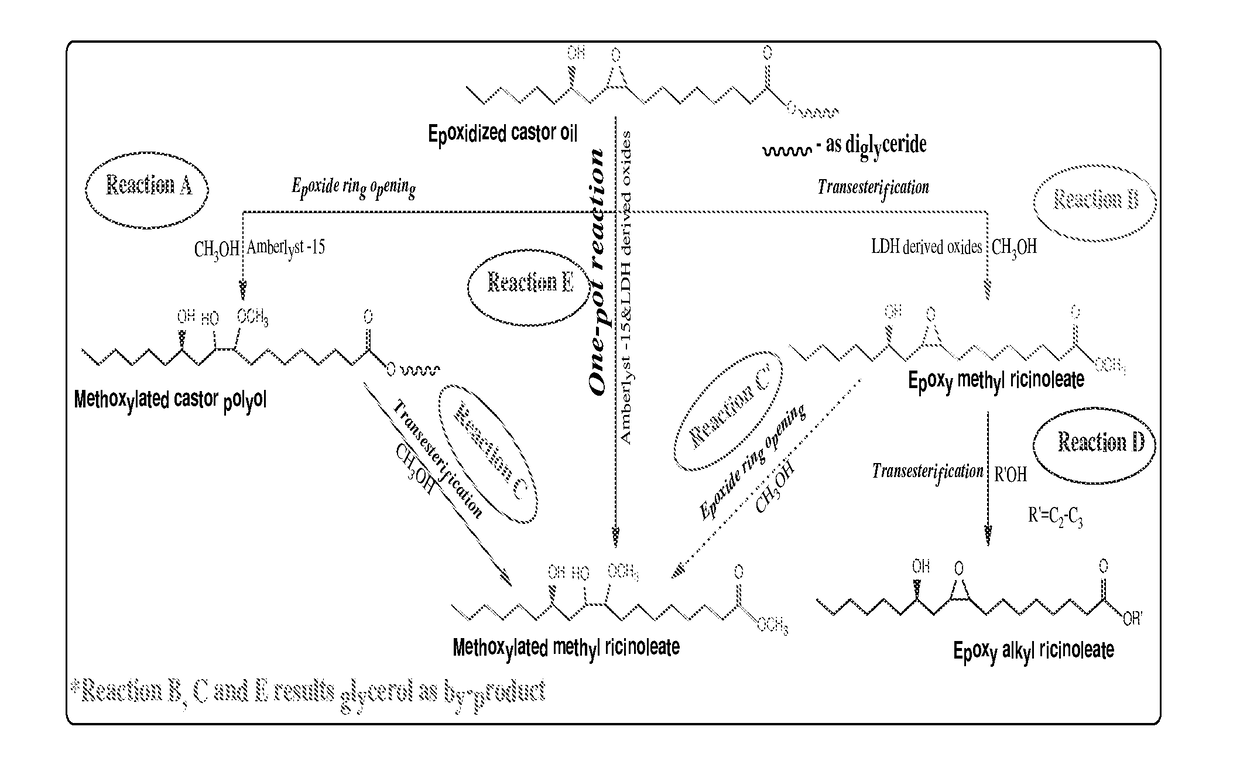
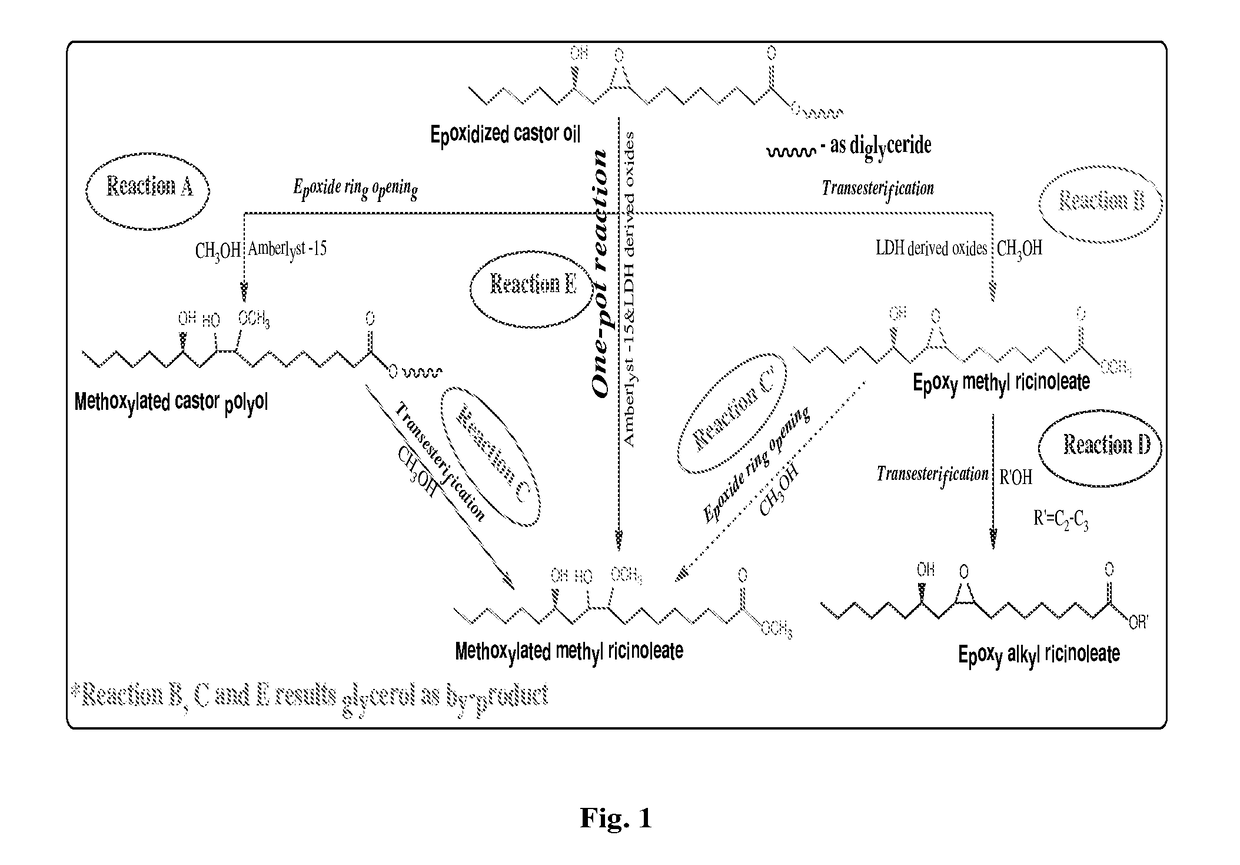
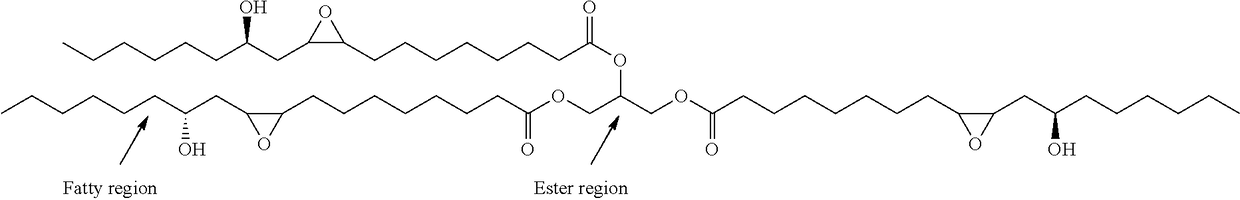
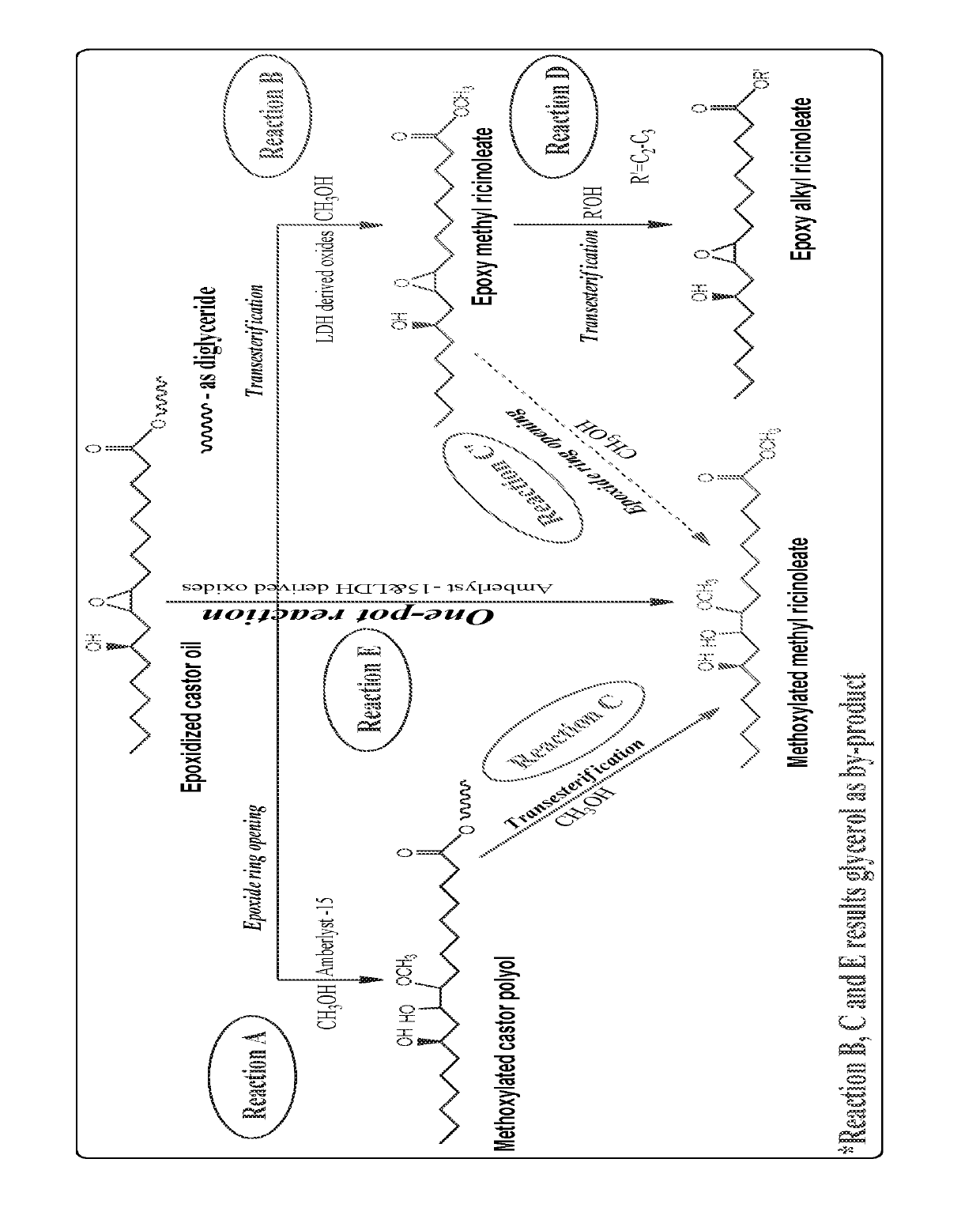
Preparation of functionalized castor oil derivatives using solid acid and base catalysts
This invention relates to the development of processes for the preparation of functionalized castor oil derivatives namely ring-opened glyceryl ricinoleates, epoxy alkyl ricinoleates and ring-opened alkyl ricinoleates with tailorable properties from epoxidized castor oil as raw material using heterogeneous acid and base catalysts. More particularly, the invention employs two reaction chemistries namely ring-opening and transesterification using Amberlyst 15 as solid acid catalyst for the former and oxides derived from CaAl layered double hydroxide (CaAl-LDH) as solid base catalyst for the latter and combinations thereof. Furthermore, both the catalysts are reusable and the products are easily separable after the reaction by simple physical processes.
Owner:COUNCIL OF SCI & IND RES
Synthetic method of diallyl diethylene glycol carbonate
InactiveCN103113231AOvercoming high temperature resistanceOvercoming activityPreparation from organic carbonatesMethyl carbonateDiethylene glycol
The invention belongs to the technical field of synthesis of diallyl diethylene glycol carbonate, and particularly relates to a synthetic method of diallyl diethylene glycol carbonate. The synthetic method comprises the following steps of: (1) adding diethylene glycol, allyl alcohol, dimethyl carbonate and catalyst ion exchange resin Amberlyst-15 into a reaction still, reacting the dimethyl carbonate with the allyl alcohol to generate diallycarbonate and methanol, carrying out an ester exchange reaction on the diallycarbonate and the diethylene glycol at 50-140 degree centigrade, reflowing and thermally insulating for 2-3 hours; and (2) distilling the reaction liquid in the step (1), recovering methanol at 65-90 DEG C, recovering allyl alcohol and diallycarbonate at 95-140 DEG C, reducing to the room temperature of the catalyst-containing mixed liquor, filtering and separating the catalyst ion exchange resin Amberlyst-15 to obtain required products. The synthetic method of diallyl diethylene glycol carbonate disclosed by the invention has the advantages of simple method, heat-resisting catalyst and high purity of the obtained products, and is capable of saving the production cost.
Owner:山东鲁源化工科技有限公司
A kind of technique of catalytically synthesizing tert-butyl acrylate
InactiveCN104844455BSimple stepsHigh yieldOrganic compound preparationCarboxylic acid esters preparationFiltrationAmberlyst-15
The invention relates to a method used for catalyzed synthesis of tert-butyl acrylate. The method comprises following steps successively:1) acrylic acid, a catalyst combination, a polymerization inhibitor A, and a polymerization inhibitor B are delivered into a sealed reaction kettle, and liquefied isobutylene is added dropwise for esterification, wherein the catalyst combination is composed of nanosolid superacid SO<4><2-> / SnO<2>, Amberlyst-15, D001 resin, and NKC-9 resin; 2) after adding, heat preservation is carried out; 3) after heat preservation, the sealed reaction kettle is subjected to pressure relief, an obtained mixture is subjected to filtration, and an obtain liquid ingredient is pumped into a rectifying tower; and 4) by-products tert-butyl alcohol, diisobutylene, and the target product tert-butyl acrylate are obtained via separation successively. Yield of tert-butyl acrylate is higher than 98%; reuse ratio of the catalyst is high; the method is simple; and no pollution is caused.
Owner:徐德良 +1
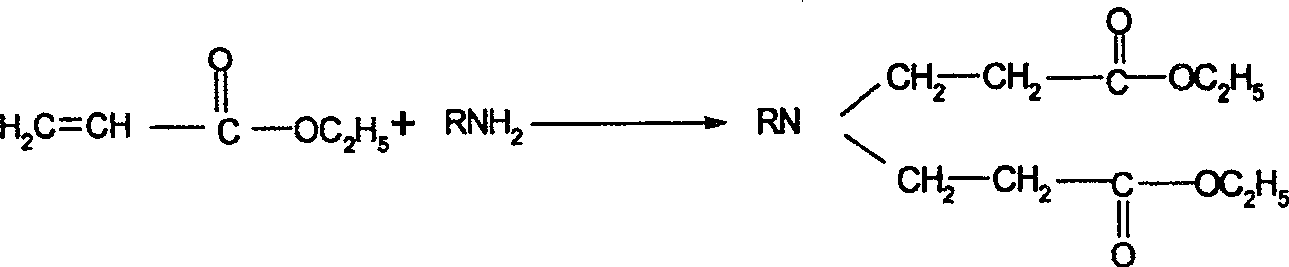
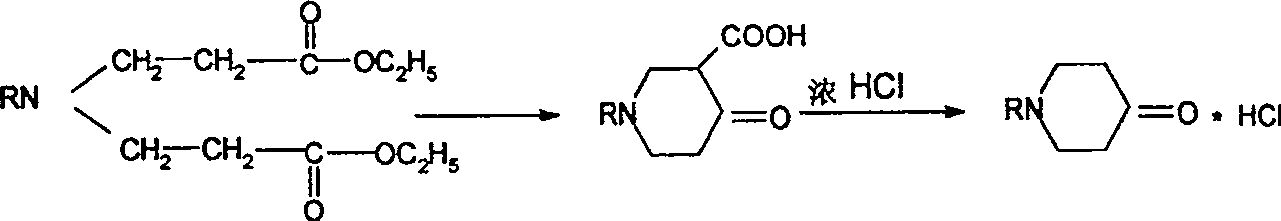
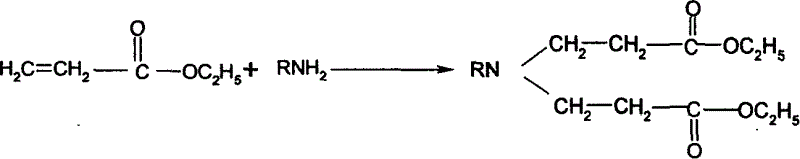
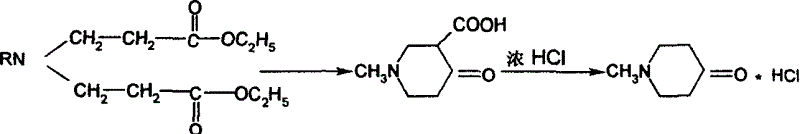
Synthesis process of N-sustituent-4-piperidyl alcohol
The synthesis process of preparing N-substituent-4-piperidyl alcohol includes the following steps: 1. adding primary amine into ethyl acrylate to react to obtain the secondary addition product; 2. adding cyclizing agent into the secondary addition product and adding concentrated hydrochloric acid after finishing cyclization to obtain N-substituent-4-piperidone hydrochloride; 3. reducing N-substituent-4-piperidone hydrochloride with NaBH4-Amberlyst-15(H+) system and regulating pH value to extract N-substituent-4-piperidyl alcohol; and 4. recovering and treating resin for reuse. The present invention has wide raw material resource, mild reaction condition, simple operation, low production cost, high yields in different steps, and excellent industrial application foreground.
Owner:SOUTHEAST UNIV
Synthesis process of N-sustituent-4-piperidyl alcohol
The synthesis process of preparing N-substituent-4-piperidyl alcohol includes the following steps: 1. adding primary amine into ethyl acrylate to react to obtain the secondary addition product; 2. adding cyclizing agent into the secondary addition product and adding concentrated hydrochloric acid after finishing cyclization to obtain N-substituent-4-piperidone hydrochloride; 3. reducing N-substituent-4-piperidone hydrochloride with NaBH4-Amberlyst-15(H+) system and regulating pH value to extract N-substituent-4-piperidyl alcohol; and 4. recovering and treating resin for reuse. The present invention has wide raw material resource, mild reaction condition, simple operation, low production cost, high yields in different steps, and excellent industrial application foreground.
Owner:SOUTHEAST UNIV
Method for reinforcing oleophobic property of catalyst surface
InactiveCN1803289AExtended service lifeHigh selectivityCatalyst protectionCatalyst activation/preparationSolid acidSilica gel
The invention discloses an oleophobic property reinforcing method of catalyst surface, which comprises the following steps: coating certain acid catalyst with oleophobic property such as Teflonx-{[(HSiW) / (SiO2)]y)(1-x), Teflonx / Amberlyst-15 and Teflonx / Amberlyst-35; detecting the finishing and finished touch corner of solid acid surface and soybean oil drip through droplet shape analyzer.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Method for synthesizing diethoxymethane from dimethoxymethane and ethanol
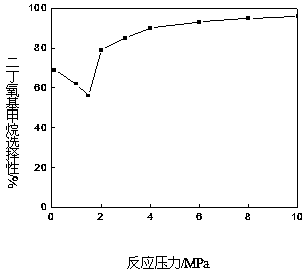
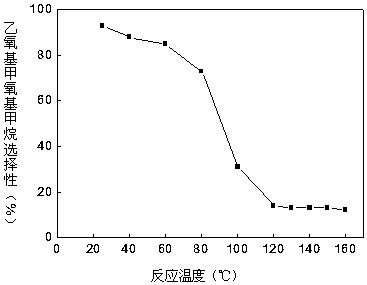
ActiveCN105669393AHigh selectivityEasy to operateOrganic chemistryOrganic compound preparationReaction temperatureAmberlyst-15
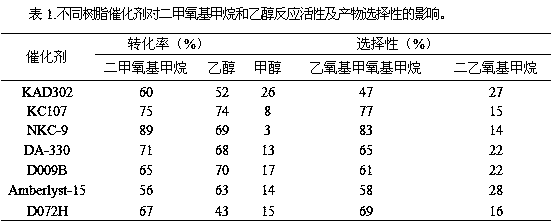
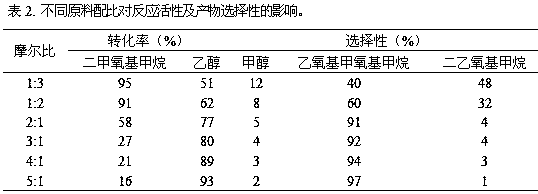
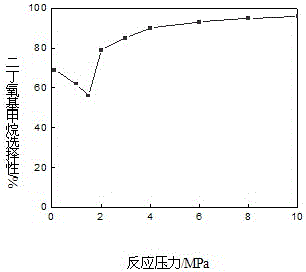
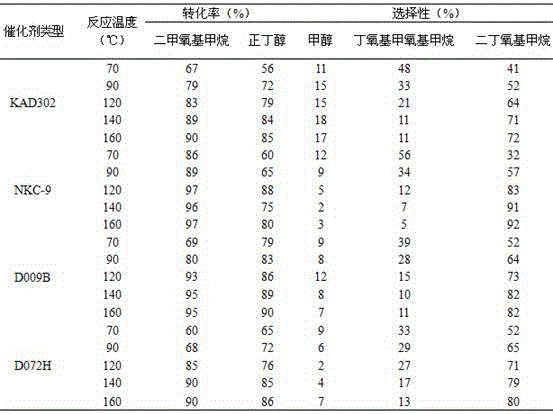
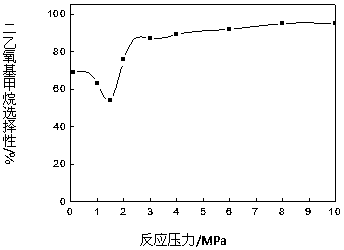
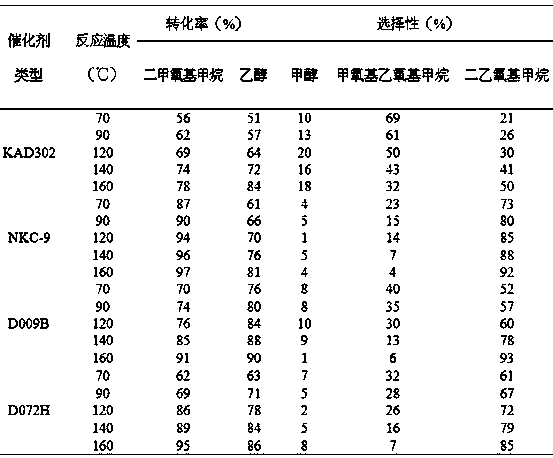
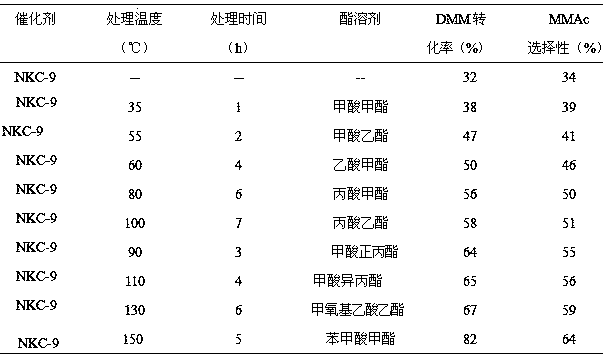
The invention provides a method for synthesizing diethoxymethane from dimethoxymethane and ethanol, relating to a method for preparing methane. The method comprises the following step: by using dimethoxymethane and ethanol as raw materials, reacting at 0-160 DEG C under the reaction pressure of 0.1-10.0 MPa by using a liquid acid and a supported liquid acid as catalysts to generate diethoxymethane at high selectivity. The resin catalyst is one or more of KAD302, KC107, NKC-9, DA-330, D009B, Amberlyst-15 and D072H containing sulfonic acid functional group. The reaction temperature is 0-160 DEG C, and the reaction pressure is 0.1-10.0 MPa. The filling gas is inert gas which is argon, helium, carbon dioxide or N2 mixture or a gas mixture thereof. The method has the advantages of single product and high selectivity, and has favorable application prospects. The required raw materials are cheap and accessible, and the whole process is simple to operate and can not generate any environment-polluting chemical substance. Thus, the method belongs to an environment-friendly technical route.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
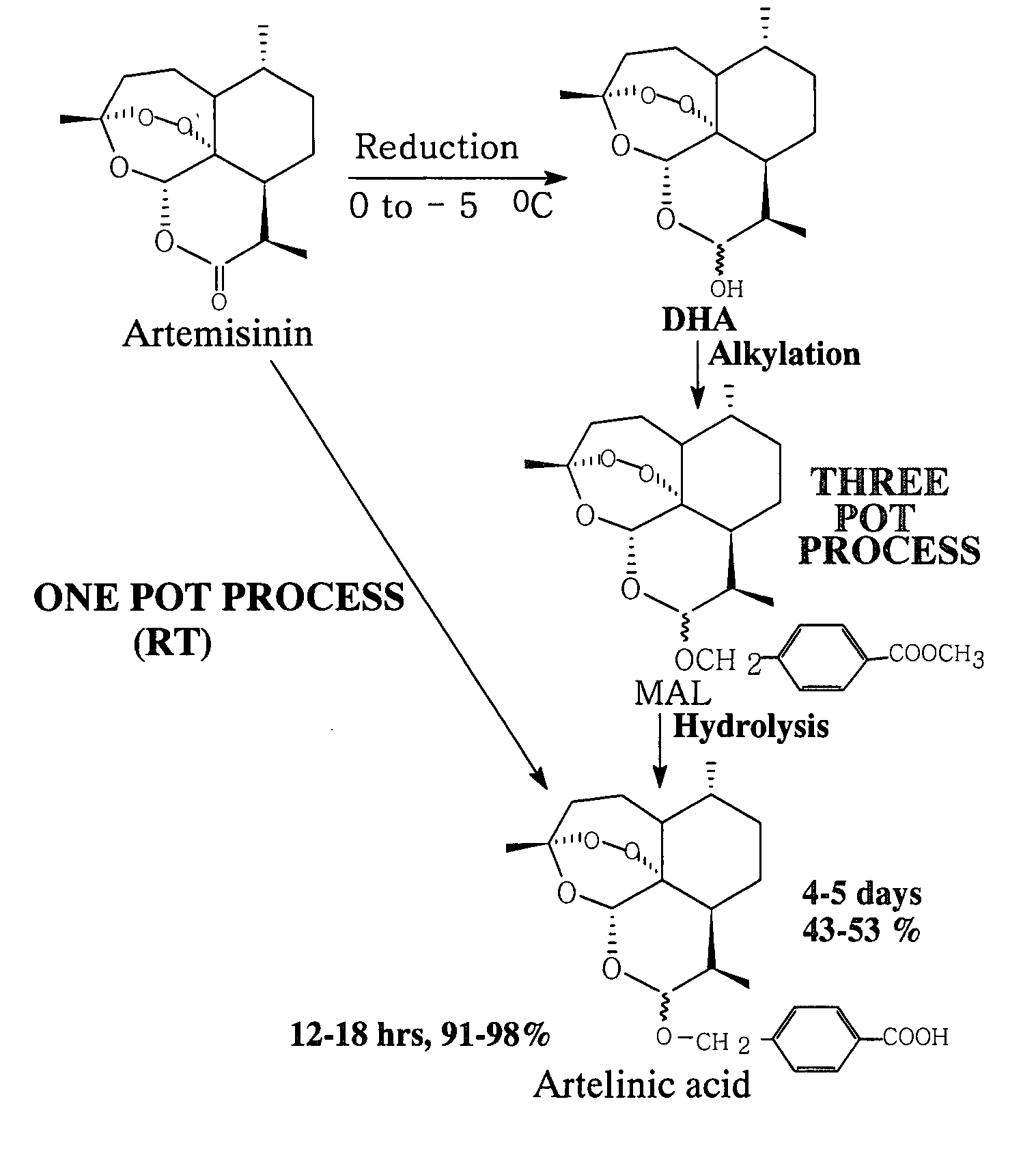
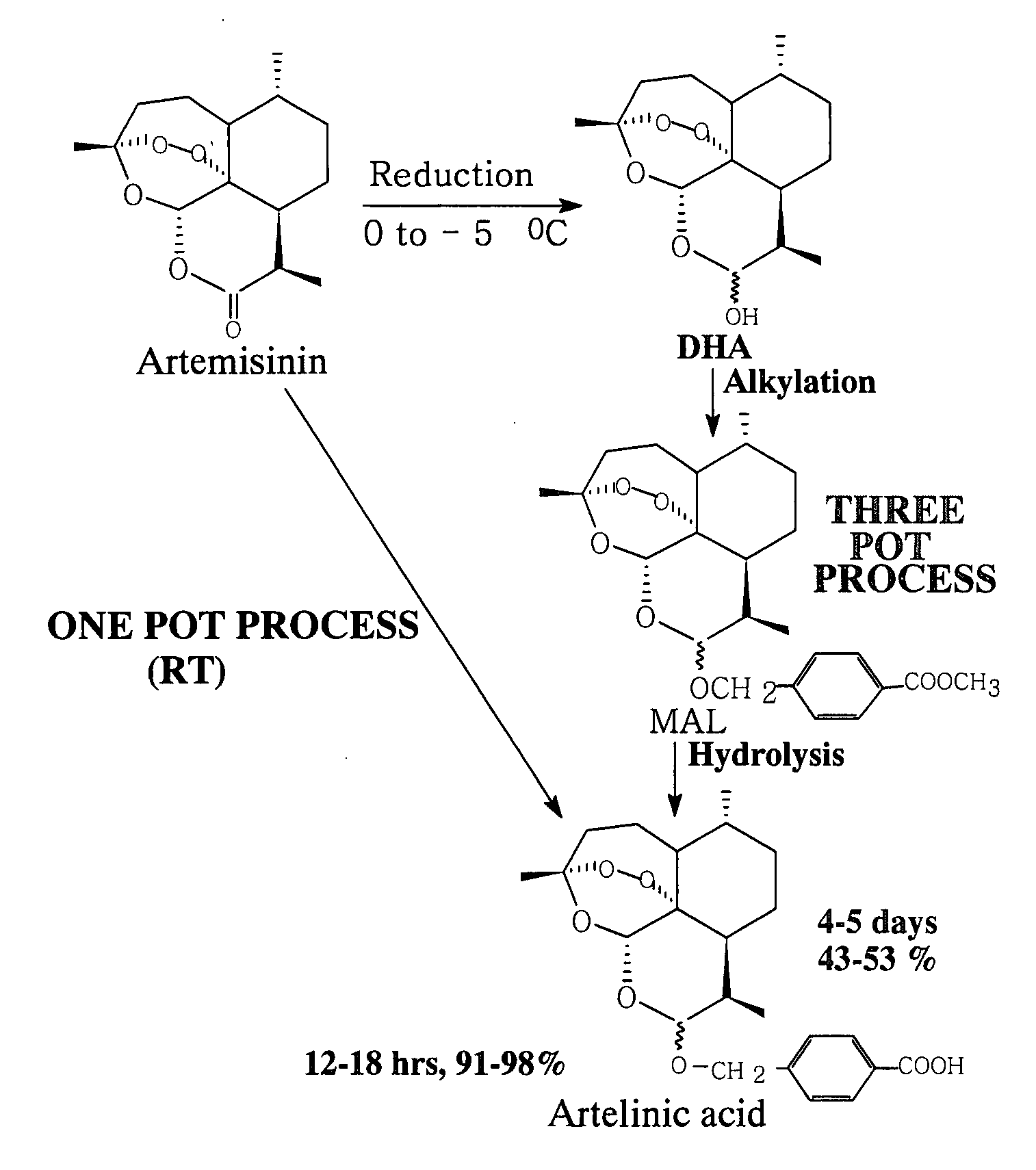
Process for one pot conversion of artemisinin into artelinic acid
The present invention relates to an improved process for one pot conversion of artemisinin into artelinic acid, which reduces the three step (three pot) conversion of artemisinin to artelinic acid in one step (one pot). The process of preparation of artelinic acid involves stirring of artemisinin with sodium borohydride, catalyst, polyhydroxy compound or chlorotrimethylsilane or amberlyst-15 resin and methyl p-(hydroxymethyl) benzoate, filtration of undissolved, unwanted reaction products and finally stirring of the filtrate with alcoholic or aqueous alkali hydroxide.
Owner:COUNCIL OF SCI & IND RES
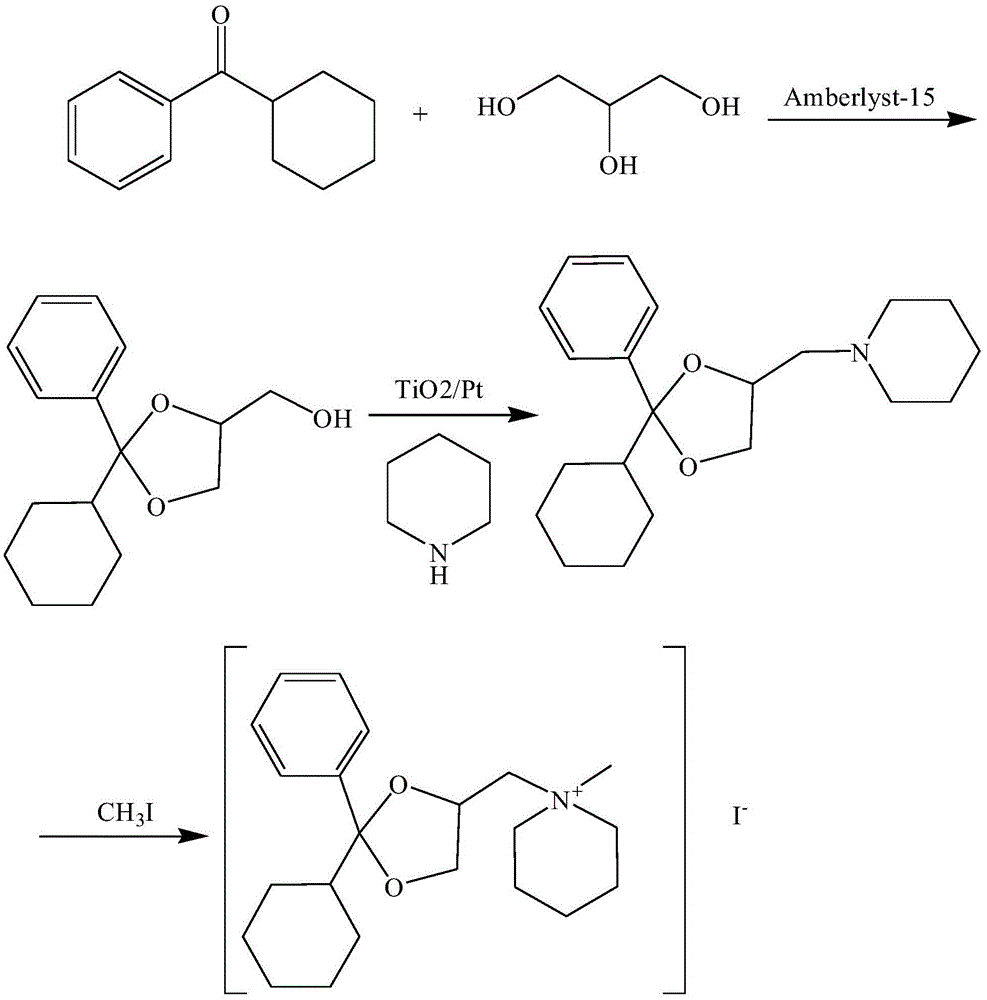
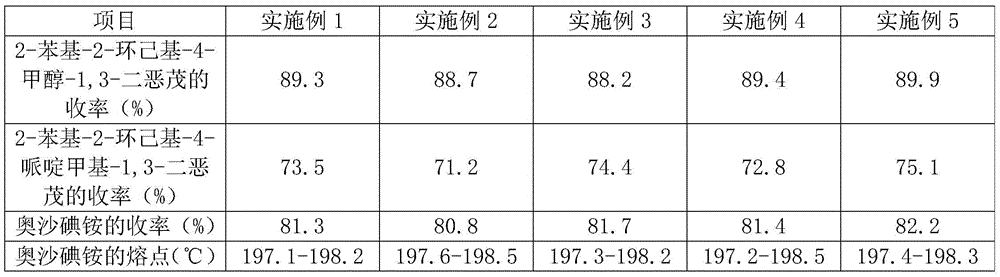
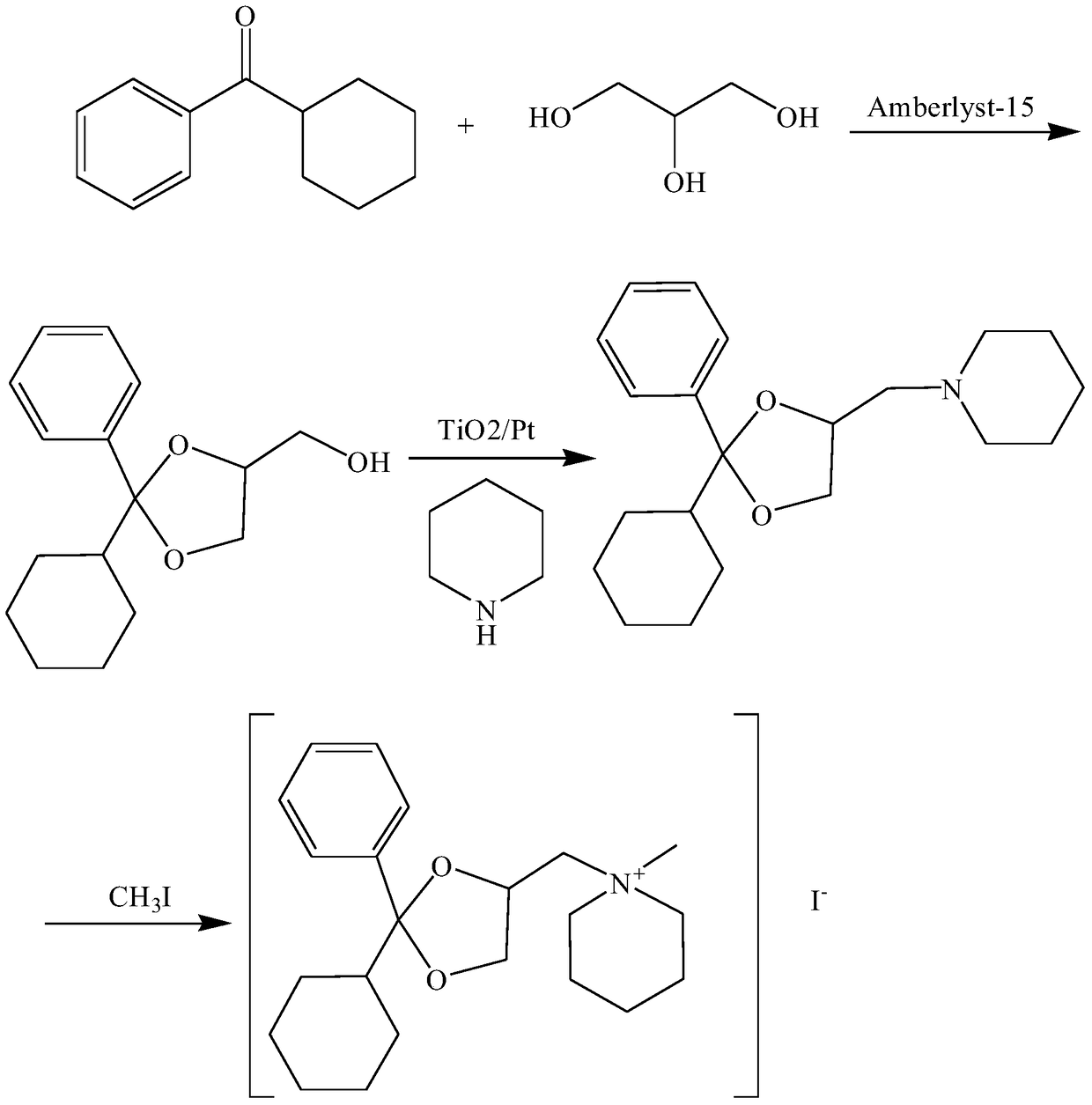
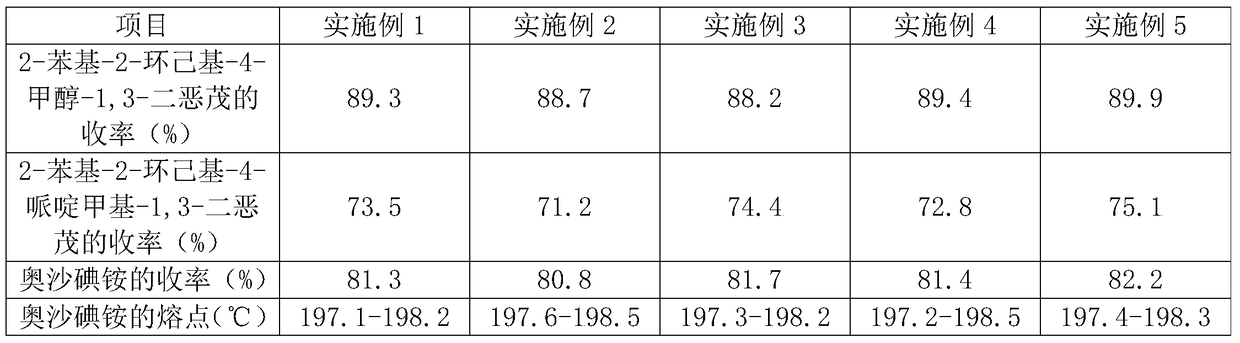
Oxapium iodide preparation method
The invention discloses an oxapium iodide preparation method. The method comprises the steps of S1, preparing 2-phenyl-2-hexamethylene-4-methanol-1,3-dioxolane, S2, preparing 2-phenyl-2-hexamethylene-4-piperidinemethyl-1, 3-dioxolane, and S3, preparing the oxapium iodide. The step S1 comprises the steps of mixing uniformly cyclohexyl phenyl ketone, glycerol, Amberlyst-15 exchange resin and allochroic silicagel, heating, maintaining the temperature, stirring, filtering, taking the filtered solution, adding saturated sodium hydrogen carbonate aqueous solution for washing, keeping static, taking organic layer, drying, releasing pressure, distilling to acquire material A, purifying material A, drying to acquire 2-phenyl-2-hexamethylene-4-methanol-1, 3-dioxolane. The method has the advantages of being mild in reaction condition, simple in operation, suitable for industrialized production, low in material toxicity, easy and cheap for material access, low in cost and good in comprehensive yield rate.
Owner:HEFEI PINGGUANG PHARMA
A kind of method for preparing dibutoxymethane with dimethoxymethane and n-butanol as raw material
InactiveCN105801386BHigh selectivityRaw materials are cheap and easy to getOrganic chemistryOrganic compound preparationReaction temperatureN-Butanol
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Preparation of quinoxaline derivatives
InactiveCN101481357BNot easy to cause pollutionShorten the timeAntibacterial agentsOrganic chemistryQuinoxalineOrganic solvent
The invention discloses a preparation method of a quinoxaline derivative. The method is characterized by condensing an o-diamino compound with an o-dicarbonyl compound in a polar solvent in the presence of catalyst Amberlyst-15 or NKC-9 dry hydrogen catalyzed resin Styrene-DVB to prepare the quinoxaline derivative. The preparation method takes ordinary tap water instead of a toxic organic solventas the solvent for a condensation reaction to obtain the quinoxaline derivative with high yield and high content. The catalyst can be recovered and can be reused for at least three times in a condition of no loss of the catalytic activity, thus effectively reducing the production cost. The preparation method of the quinoxaline derivative has the advantages of simple process operation, no environment pollution, and is fit for large-scale industrialized production.
Owner:TIANJIN NORMAL UNIVERSITY
A kind of preparation method of ethoxymethoxymethane
InactiveCN105585463BHigh selectivityNo pollutionOrganic chemistryOrganic compound preparationFixed bedReaction temperature
A method for preparing ethoxymethoxymethane, relates to a method for preparing methane, the method uses dimethoxymethane and ethanol as raw materials, and uses resin as a catalyst under temperature and pressure to prepare ethoxymethane Resin catalyst is one or more resin catalysts of KAD302, KC107, NKC-9, DA-330, D009B, Amberlyst-15, D072H; Resin catalyst is based on the resin catalyst containing sulfonic acid group; The reaction temperature is 0-160°C, the reaction pressure is 0.1-10.0 MPa; the filling gas is an inert gas, which is one of argon, helium, carbon dioxide, or nitrogen or a mixture thereof; the reactor is a fixed bed or tank type Reactor; the molar ratio of raw materials dimethoxymethane and ethanol is 1:2 to 5:1. The synthesis process of the invention has simple products, fewer side reactions, and high selectivity of the required product ethoxymethoxymethane; meanwhile, the method of the invention basically does not pollute the environment.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method for preparing dibutoxymethane from dimethoxymethane and n-butyl alcohol
InactiveCN105801386AHigh selectivityRaw materials are cheap and easy to getOrganic chemistryOrganic compound preparationReaction temperatureDimethoxymethane
The invention provides a method for preparing dibutoxymethane from dimethoxymethane and n-butyl alcohol and relates to a method for preparing dibutoxymethane. The method comprises steps as follows: dimethoxymethane and n-butyl alcohol which serve as raw materials are subjected to a reaction in the presence of catalysts, namely, liquid acid and supported liquid acid, at the reaction temperature of 0-160 DEG C under the reaction pressure of 0.1-10.0 MPa, and dibutoxymethane is produced in a high-selective manner; a resin catalyst is one or more of KAD302, KC107, NKC-9, DA-330, D009B, Amberlyst-15, D072H and the like which contain sulfonic acid functional groups; the reaction temperature is 0-160 DEG C and the reaction pressure is 0.1-10.0 MPa. According to the method, a single product is produced, the selectivity is high, required raw materials are low in price and easy to obtain, the operation is simple in the whole procedure, no chemical substances polluting the environment are produced, and the method is an environment-friendly technological process.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method for reinforcing oleophobic property of catalyst surface
InactiveCN100393417CExtended service lifeHigh selectivityCatalyst protectionCatalyst activation/preparationSolid acidSilica gel
The invention discloses an oleophobic property reinforcing method of catalyst surface, which comprises the following steps: coating certain acid catalyst with oleophobic property such as Teflonx-{[(HSiW) / (SiO2)]y)(1-x), Teflonx / Amberlyst-15 and Teflonx / Amberlyst-35; detecting the finishing and finished touch corner of solid acid surface and soybean oil drip through droplet shape analyzer.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
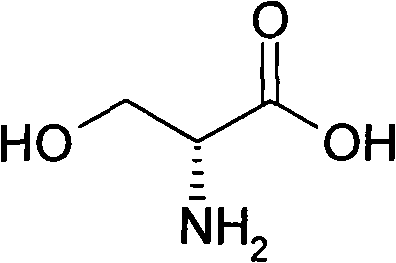
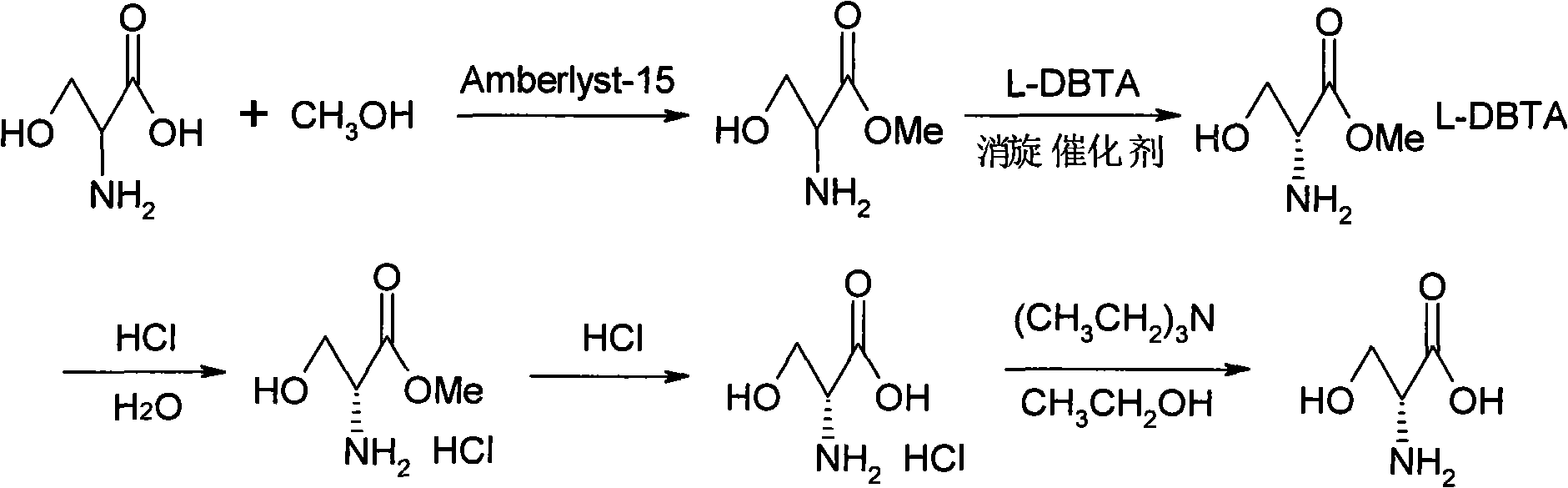
Method for preparing D-serine by kinetic resolution
ActiveCN101735085BHigh split yieldReduce operating costsOrganic compound preparationAmino-carboxyl compound preparationSerine methyl esterKinetic resolution
The invention discloses a method for preparing D-serine by kinetic resolution. The method takes DL-serine as raw material and is implemented by esterifying DL-serine with methanol under catalysis of Amberlyst-15 ion exchange resin to obtain DL-serinemethylester, carrying out dynamic kinetic resolution on DL-serinemethylester and resolving agent L-DBTA under action of racemization catalyst to obtain dibasic DL-serinemethylester.L-DBTA, dissociating with hydrochloric acid, and hydrolyzing to obtain D-serine, the target product of the invention. Compared with the prior art, by using the racemization catalyst in the invention, the dynamic continuous conversion of dibasic L-serinemethylester.L-DBTA into dibasic DL-serinemethylester.L-DBTA can be realized with the theoretic conversion rate being approximate to 100%, thus greatly enhancing yield of resolution, reducing operation cost, stabilizing product quality and being suitable for industrialized production.
Owner:SHANGHAI SHISI CHEM PROD
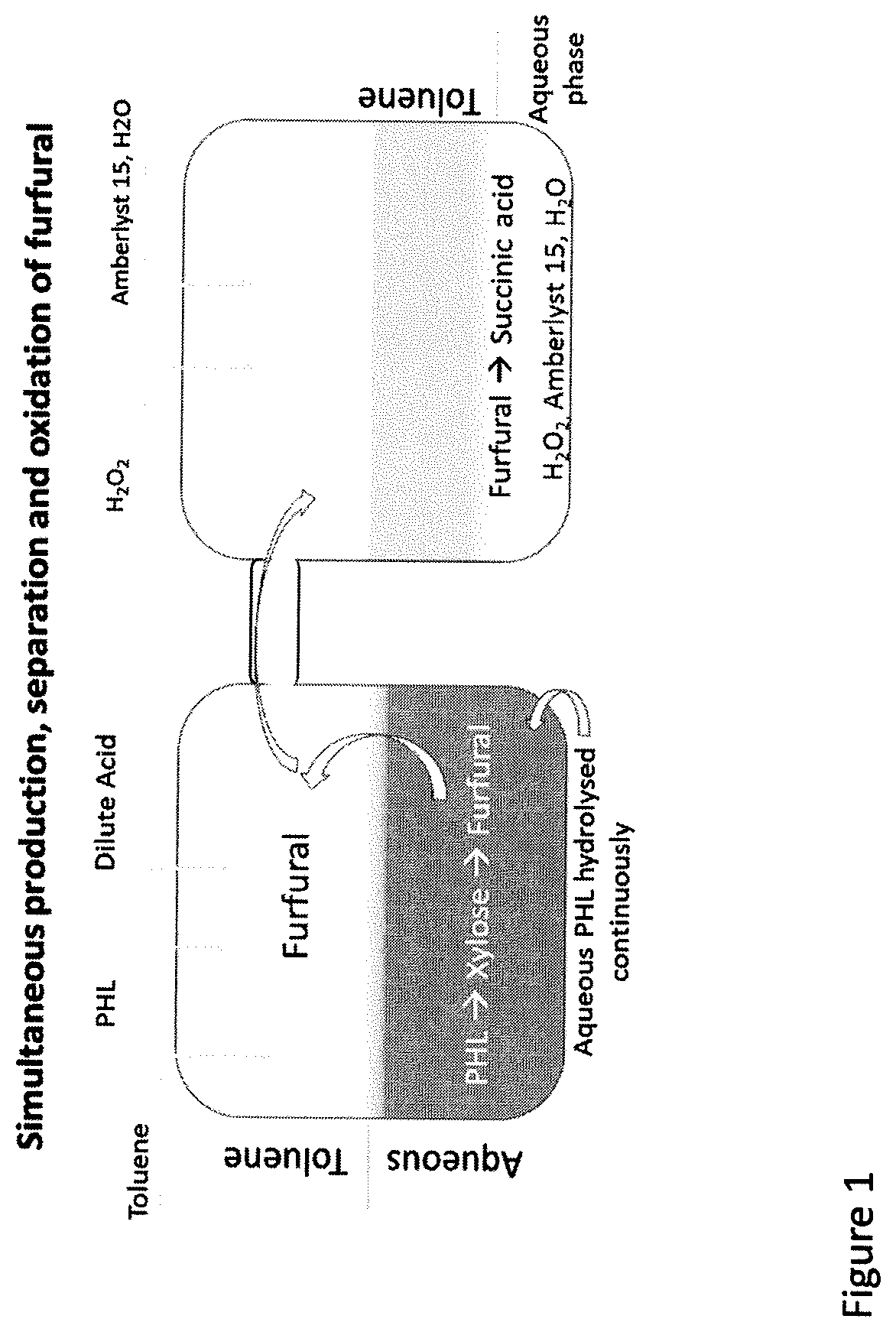
Conversion of Wood Based Hemicellulose Prehydrolysate into Succinic Acid Using a Heterogeneous Acid Catalayst in a Biphasic System
ActiveUS20190382328A1Low water solubilityCation exchanger materialsOrganic-compounds/hydrides/coordination-complexes catalystsOrganic solventHydrolysate
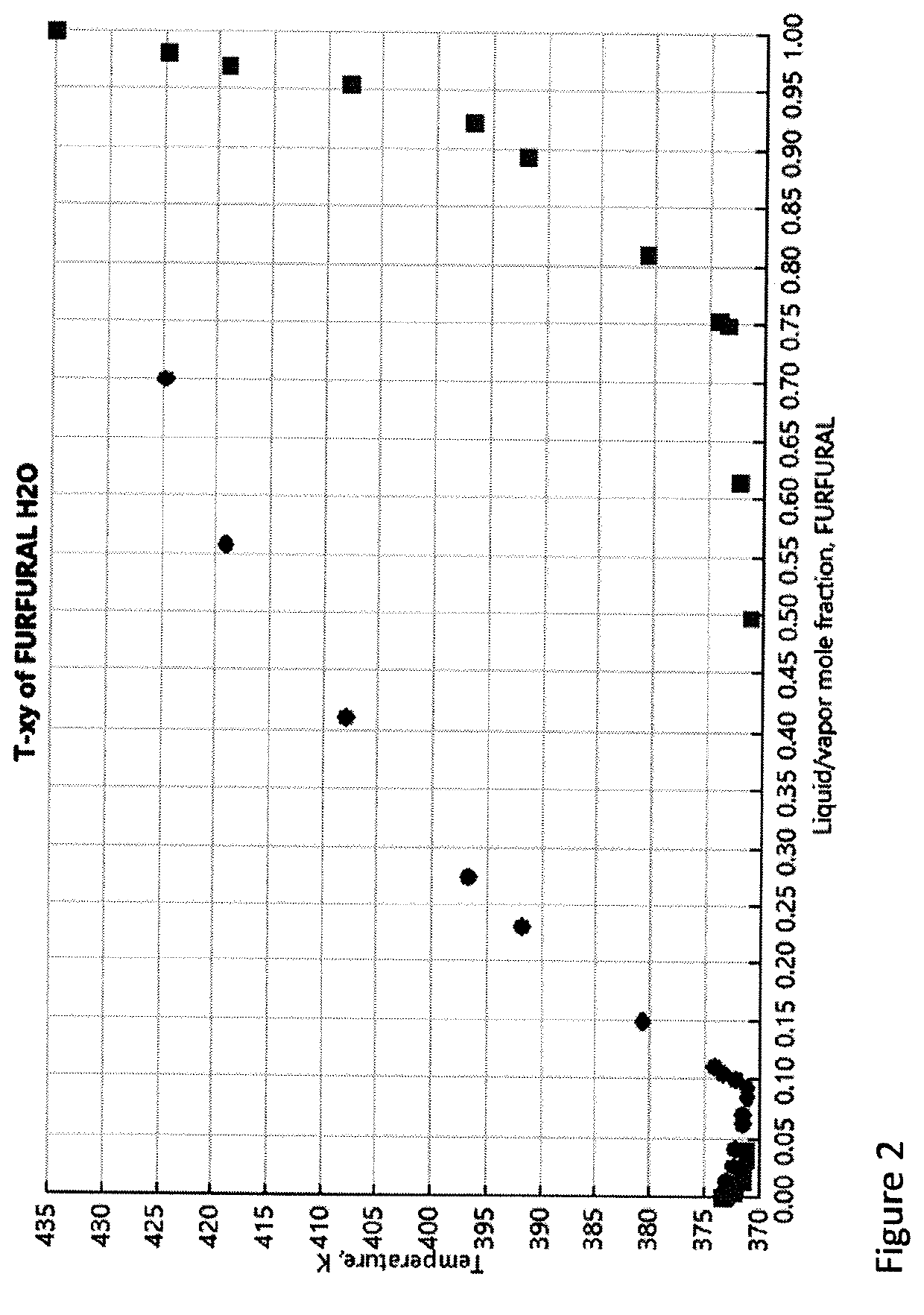
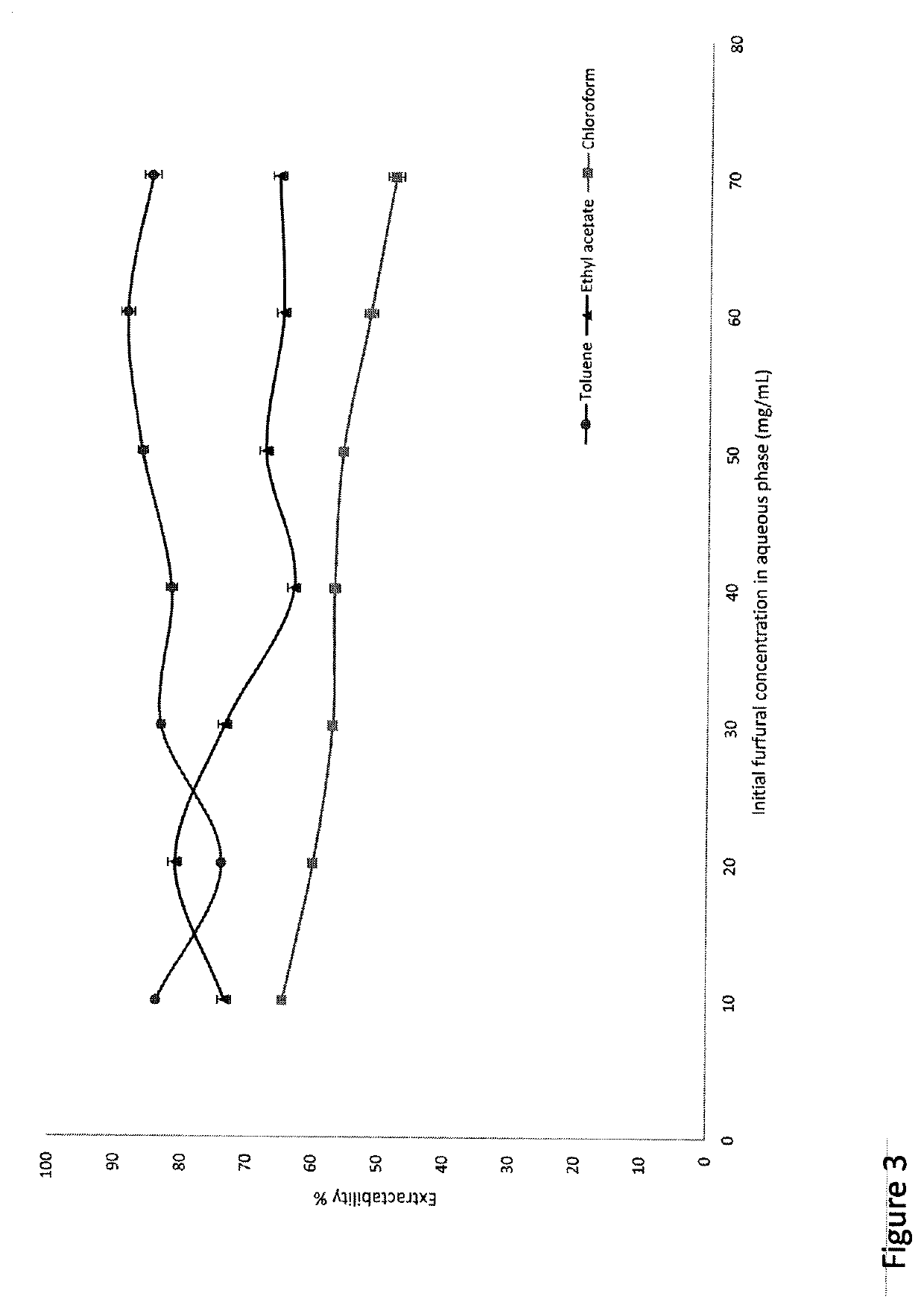
A novel approach for the conversion of biomass based hemicellulose prehydrolysate to high value succinic acid has been investigated using a heterogeneous acid catalyst, Amberlyst 15 and hydrogen peroxide. A vital intermediate in this process, furfural, was oxidized in a biphasic system to produce succinic acid. Production of furfural in good yields is a limiting step in such processes for a number of reasons. Among the organic solvents evaluated, toluene was found to be an ideal solvent for furfural extraction and facilitated the conversion of furfural to succinic acid. Simultaneous extraction of furfural into the organic solvent as it is produced, increased the overall yield. It was observed that the developed method resulted in a succinic acid yield of 49% from the furfural obtained from hemicellulose prehydrolysate. It was found that 50 mg of Amberlyst 15 per mmole of furfural resulted in 100% FA conversion in less time.
Owner:LAKEHEAD UNIVERSITY
A method for synthesizing diethoxymethane with dimethoxymethane and ethanol
ActiveCN105669393BHigh selectivityEasy to operateOrganic chemistryOrganic compound preparationReaction temperatureAmberlyst-15
A method for synthesizing diethoxymethane with dimethoxymethane and ethanol relates to a method for preparing methane, using dimethoxymethane and ethanol as raw materials, using liquid acid and loaded liquid acid as catalysts, and using dimethoxymethane and ethanol as catalysts. React under the conditions of reaction temperature 0-160°C and reaction pressure 0.1-10.0 MPa to form diethoxymethane with high selectivity; resin catalysts are KAD302, KC107, NKC‑9, DA‑330, D009B, Amberlyst containing sulfonic acid functional groups ‑15, D072H one or more resin catalysts; the reaction temperature is 0-160°C, the reaction pressure is 0.1-10.0 MPa; the filling gas is an inert gas, which is one of argon, helium, carbon dioxide, or N2 mixed gas species or mixture. The product of the invention is relatively single, has high selectivity, and has good application prospects. Moreover, the raw materials required by the method are cheap and easy to obtain, and the whole process is easy to operate. At the same time, the method does not produce any chemical substances that pollute the environment, and belongs to an environmentally friendly process path.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Preparation of functionalized castor oil derivatives using solid acid and base catalysts
This invention relates to the development of processes for the preparation of functionalized castor oil derivatives namely ring-opened glyceryl ricinoleates, epoxy alkyl ricinoleates and ring-opened alkyl ricinoleates with tailorable properties from epoxidized castor oil as raw material using heterogeneous acid and base catalysts. More particularly, the invention employs two reaction chemistries namely ring-opening and transesterification using Amberlyst 15 as solid acid catalyst for the former and oxides derived from CaAl layered double hydroxide (CaAl-LDH) as solid base catalyst for the latter and combinations thereof. Furthermore, both the catalysts are reusable and the products are easily separable after the reaction by simple physical processes.
Owner:COUNCIL OF SCI & IND RES
A kind of synthetic method of high 4,4'-isomer content bisphenol f
InactiveCN105037107BSuitable for continuous productionLow reaction temperatureOrganic chemistryOrganic compound preparationSynthesis methodsReaction temperature
The invention discloses a synthesis method of high-4,4'-isomer-content bisphenol F, which comprises the following step: by using phenol and formaldehyde as raw materials, synthesizing bisphenol F by a two-step process by using Amberlyst-36 and Amberlyst-15 cation exchange resins as catalysts, wherein the mass percent of the 4,4'-isomer bisphenol F is up to 60% or above, and the mass yield of the bisphenol F is up to 90% or above. The catalysts are commercial cation exchange resins, can be easily purchased, separated and recovered, and are recyclable. The method has the advantages of low reaction temperature, short reaction time and no corrosiveness to equipment, is suitable for continuous production of tubular reactors, and has favorable industrial application prospects.
Owner:XIANGTAN UNIV
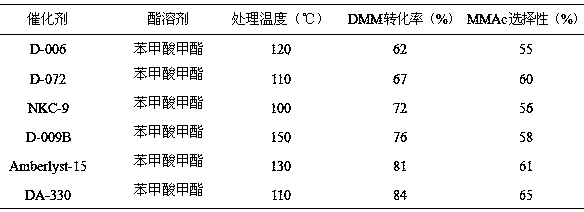
A kind of method utilizing ester solvent to process polystyrene sulfonic acid resin catalyst
InactiveCN105833905BGood choiceHigh reactivityOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationMethoxyacetic acidOrganic Ester
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
A kind of preparation method of oxalum iodide
The invention discloses an oxapium iodide preparation method. The method comprises the steps of S1, preparing 2-phenyl-2-hexamethylene-4-methanol-1,3-dioxolane, S2, preparing 2-phenyl-2-hexamethylene-4-piperidinemethyl-1, 3-dioxolane, and S3, preparing the oxapium iodide. The step S1 comprises the steps of mixing uniformly cyclohexyl phenyl ketone, glycerol, Amberlyst-15 exchange resin and allochroic silicagel, heating, maintaining the temperature, stirring, filtering, taking the filtered solution, adding saturated sodium hydrogen carbonate aqueous solution for washing, keeping static, taking organic layer, drying, releasing pressure, distilling to acquire material A, purifying material A, drying to acquire 2-phenyl-2-hexamethylene-4-methanol-1, 3-dioxolane. The method has the advantages of being mild in reaction condition, simple in operation, suitable for industrialized production, low in material toxicity, easy and cheap for material access, low in cost and good in comprehensive yield rate.
Owner:HEFEI PINGGUANG PHARMA
A kind of method that solid acid catalysis high yield prepares levulinic acid ester
ActiveCN103408422BHigh yieldCatalytic conversionOrganic compound preparationCarboxylic acid esters preparationFood additiveMolecular sieve
The invention discloses a high-yield levulinic acid ester preparation method under catalysis of solid acid and belongs to the field of organic synthesize. The method comprises the following steps: uniformly mixing 1 part of sugar, 10-100 parts of alcohol, 0.01-0.2 part of acid B and 0.1-1 part of acid L, and performing reaction under the temperature of 130-190 DEG C for 1-20 h to obtain a levulinic acid methyl ester solution, wherein the acid L is stanniferous molecular sieve, and the acid B is SBA-15-SO3H, Carbon-SO3H or Amberlyst-15. According to the method, the carbohydrate is low in cost and easy to obtain; with the acid B and acid L which can be used repeatedly, the catalysis effect basically remains the same; the levulinic acid ester compound prepared in one step can be widely applied to industries of food additives, essence spices, chemical reaction intermediates, gasoline, diesel-dopes and the like.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com