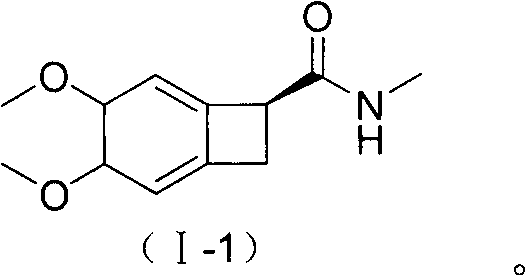
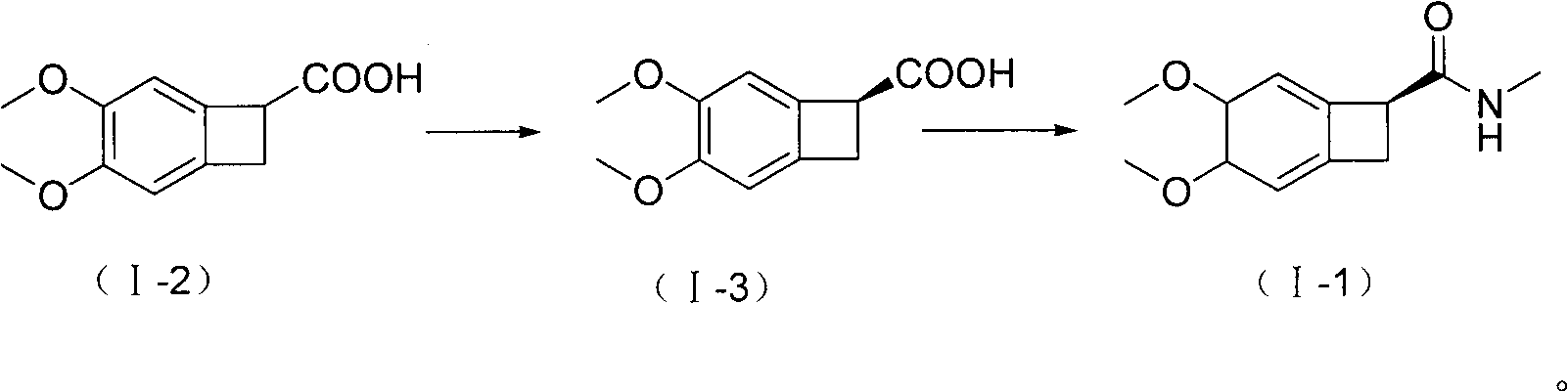
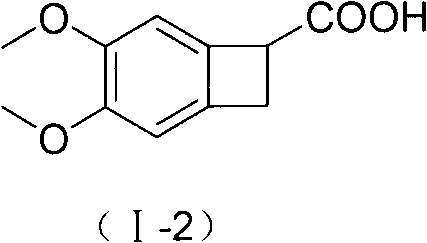
New benzocyclobutane, preparation method thereof and application thereof
A compound, the technology of I-1, is applied in the new field of benzocyclobutane, which can solve the problems that the yield has not been further improved, the mother liquor cannot be racemized and recycled, and the difficulty of large-scale production, etc., to avoid danger And it is not easy to industrialize, avoid waste and waste to the environment, and the effect of stable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0048] Embodiment (1S)-4, the synthesis of 5-dimethoxy-1-(methylaminomethyl)-benzocyclobutane oxalate
[0049] Step A: Synthesis of I-2 (4,5-dimethoxy-1-carboxy-benzocyclobutane)
[0050] 4,5-dimethoxy-1-cyano-benzocyclobutane (200g, 1.04mol), potassium hydroxide (1000g, 18mol) and ethanol (4000ml) were mixed, stirred and refluxed for 5 hours, and the reaction ended . Cool down to room temperature naturally, add water (10 L) to dissolve, evaporate ethanol under reduced pressure, acidify with concentrated hydrochloric acid, extract with ethyl acetate (1000ml×3), combine the extracts, dry over anhydrous sodium sulfate, decolorize with activated carbon, evaporate the solvent under reduced pressure, Washed with ethyl acetate (600ml) and dried to obtain 208g of the title compound with a yield of 95%. mp 137-138°C. TLC: dichloromethane:methanol=10:1, Rf value=0.4.
[0051] Step B: Synthesis of I-3 ((1S)-4,5-dimethoxy-1-carboxy-benzocyclobutane)
[0052] Step B-1: Mix the compou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com