Enzymatic resolution method of isavuconazole intermediate
A technology for the separation of isavuconazole intermediates and enzymatic methods, which is applied in fermentation and other directions, can solve the problems of poor reproducibility and inability to crystallize, and achieve simple operation, mild enzymatic separation conditions, and high chiral purity of products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
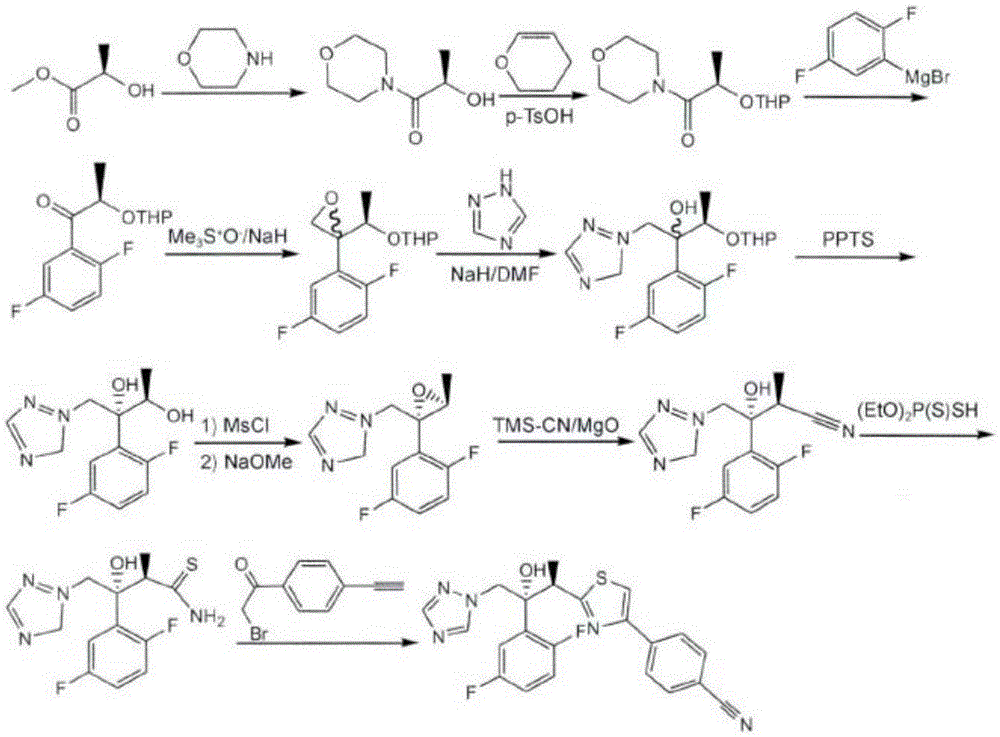
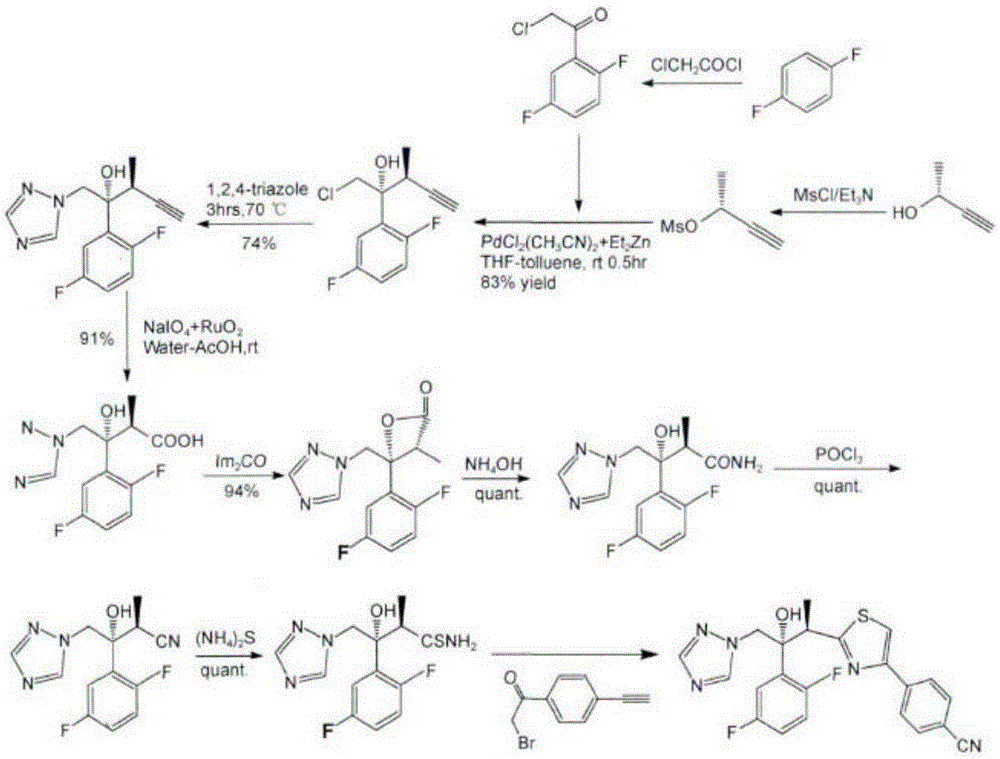
Image
Examples
Embodiment 1
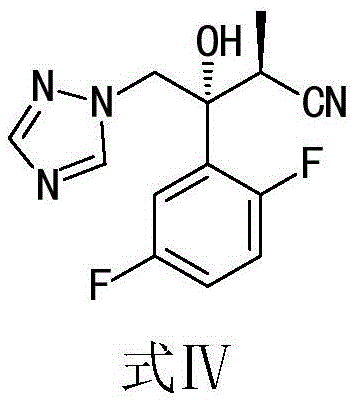
[0028] Add 100.0 g of racemic isavuconazole intermediate I, the compound of formula I, into a 500 ml flask, then add 400 ml of ethyl acetate, heat to 60°C and stir for 20 minutes, cool to room temperature, and filter to obtain filtrate A. Add 10g of commercially available AK226 nitrilase and 300ml of water into a 1000ml beaker, stir mechanically, slowly pour the filtrate A into the enzymolysis solution, stir slowly at 32°C, maintain a two-phase layered state, add 5% hydrogen dropwise Sodium oxide solution, control the pH value to 8-8.5. After the pH is stable and the sodium hydroxide solution is no longer consumed, the liquid is separated, the organic layer is filtered, and the solvent is evaporated under reduced pressure at 50°C to obtain the crude product of the compound (2S,3R)-intermediate I shown in formula IV. Add 60ml of methyl tert-butyl ether to the crude product of compound (2S,3R)-intermediate Ⅰ shown in formula IV, make a slurry, filter, and blow dry at 60°C to obt...
Embodiment 2
[0030] Add 1.00kg of racemic isavuconazole intermediate I, the compound of formula I, into a 5000ml flask, then add 3.0L of ethyl acetate, heat to 70°C and stir for 10 minutes, cool to room temperature, and filter to obtain filtrate A. Mix 120g of commercially available AK226 nitrilase with 2.0L of water, stir mechanically, slowly pour the filtrate A into the enzymolysis solution, stir at a slow speed at 35°C, maintain the state of two-phase separation, add saturated sodium carbonate solution dropwise, Control the pH value from 7.5 to 8.0. After the pH is stable and the sodium carbonate solution is no longer consumed, separate the layers, extract the water layer with 1.0L ethyl acetate, combine the organic layer and filter, remove the enzyme from the water layer, and evaporate the solvent under reduced pressure at 55°C to obtain the structure shown in formula IV Compound (2S,3R) - intermediate I crude product. Add 1.5L of isopropanol to the crude compound (2S,3R)-intermediate...
Embodiment 3
[0033] Add 1.00kg of racemic isavuconazole intermediate I, the compound of formula I, into a 5000ml flask, then add 5.0L of ethyl acetate, heat to 50°C and stir for 30 minutes, cool to 10°C, and filter to obtain filtrate A. Filter the recovered nitrilase stored in the buffer solution in Example 2, mix the filter cake with 3.0L water, stir mechanically, slowly pour the filtrate A into the enzymolysis solution, stir slowly at 40°C, and maintain two In the state of phase separation, a saturated 5% potassium hydroxide solution was added dropwise to control the pH value to 8.4-8.6. The structural compound (2R, 3S) shown in formula IV is monitored by HPLC-intermediate I enantiomer has been hydrolyzed and the remaining ≤0.2% (area normalization method), liquid separation, 2.0L ethyl acetate back extraction water layer, combined organic layer was filtered, the water layer was removed to recover the enzyme, and the solvent was distilled off under reduced pressure at 50°C to obtain the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com