Optical separation method substituting oxyphosphonate acetate
An optical resolution, phosphinyl-based technology, applied in the optical resolution of racemates, splitting and substituting phosphinyl acetic acid to obtain its single enantiomer, can solve the problem of high solvent consumption and poor optical resolution method It can reduce the environmental pollution, improve the separation yield, and achieve the effect of simple and efficient recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
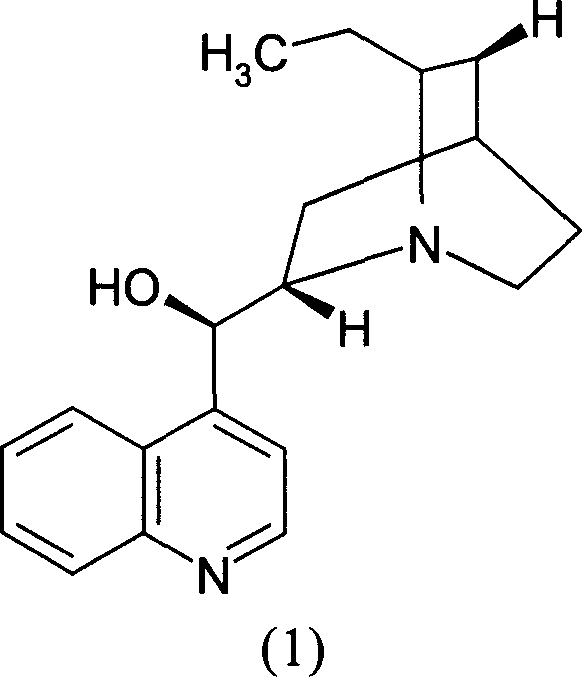
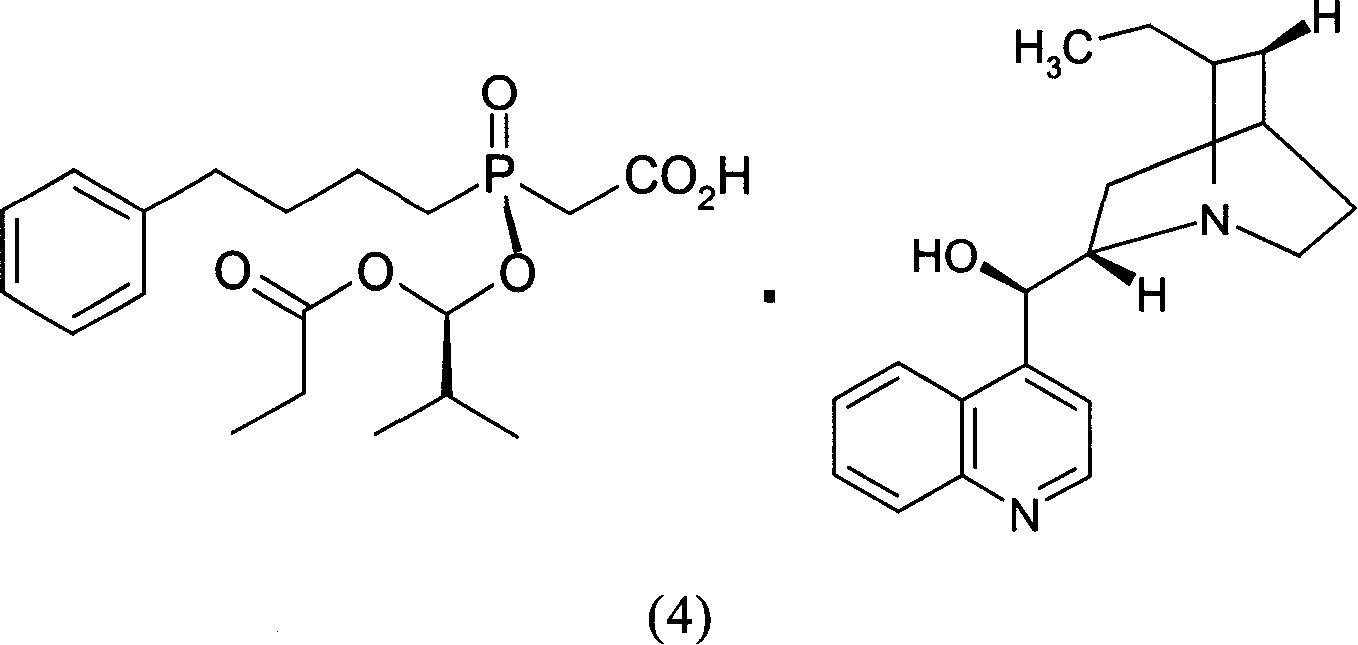
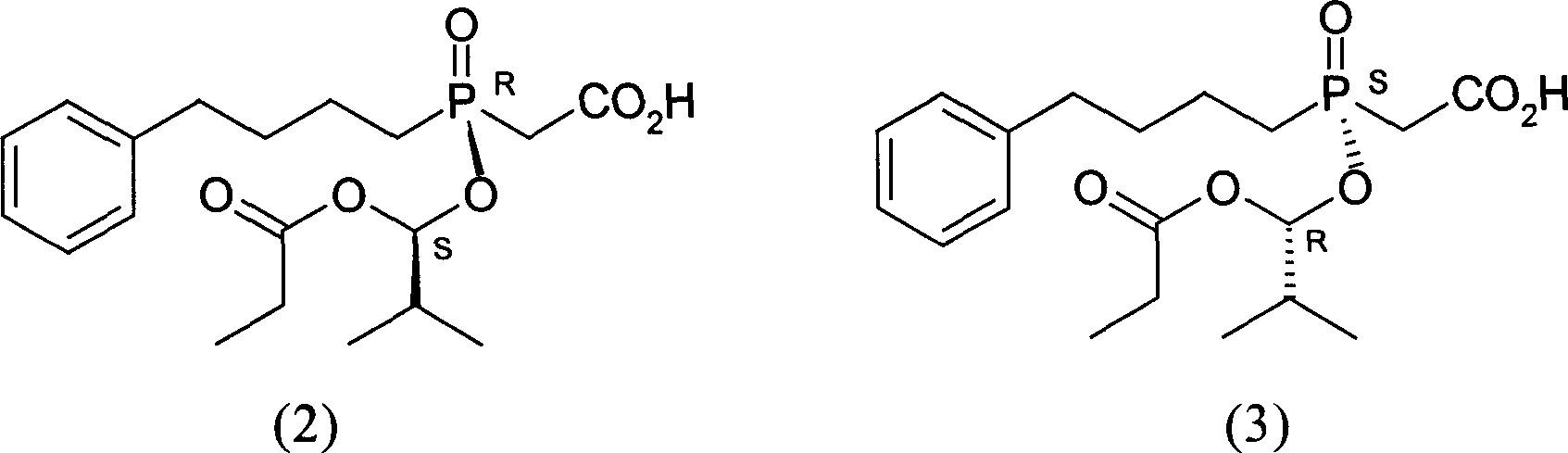
[0036] Mix and stir 10,11-dihydrocinchonidine (1) (3.56g, 12mmol) and ethyl acetate (30ml) to form a suspension, then add Base-1-(1-oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]acetic acid (2) and [(S)-[(1R)-2-methyl-1- (1-oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]racemate (7.68g, 20mmol) composed of acetic acid (3), heated to reflux with stirring until all solids Dissolved and filtered while hot. Add seed crystals to the filtrate, cool and crystallize. Suction filtration and vacuum drying gave the salt (4) generated from (2) and 10,11-dihydrocinchonidine (1) as a white solid (4.3g): melting point 125-126°C, [α] 20 D -31.7° (c=1, MeOH). A part of it was acidified and freed with dilute hydrochloric acid, and then extracted with dichloromethane to obtain oil (1): [α] 20 D +45.2° (c=1, EtOAc), the ee value determined by chiral stationary phase HPLC is 97.3%.
[0037] Get the above-mentioned salt (4) generated by (2) and 10,11-dihydrocinchonidine (1) and recrystallize on...
Embodiment 2
[0041] Mix and stir 10,11-dihydrocinchonidine (1) (7.11g, 24mmol) and ethyl acetate (68ml) to form a suspension, then add Base-1-(1-oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]acetic acid (2) and [(S)-[(1R)-2-methyl-1- (1-Oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]acetic acid (3) racemate (11.53g, 30mmol), stirred and heated until all solids were dissolved. Cool and crystallize. After suction filtration and vacuum drying, the salt (4) formed from (2) and 10,11-dihydrocinchonidine (1) was obtained as a white solid (5.88 g): melting point 122.1-125.4°C.
[0042] Get the above-mentioned salt (4) generated by (2) and 10,11-dihydrocinchonidine (1), and recrystallize once in ethyl acetate to obtain purified salt (4) produced by (2) and 10,11-dihydrocinchonidine (1). The salt (4) formed by hydrocinchonidine (1) is a white solid with a melting point of 123.4-126.5°C.
Embodiment 3
[0044] Mix and stir 10,11-dihydrocinchonidine (1) (2.96g, 10mmol) and ethyl acetate (60ml) to form a suspension, then add Base-1-(1-oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]acetic acid (2) and [(S)-[(1R)-2-methyl-1- (1-Oxopropoxy)propoxy](4-phenylbutyl)phosphinyl]acetic acid (3) racemate (7.68g, 20mmol), stirred and heated until all solids were dissolved. Cool and crystallize. Suction filtration and vacuum drying gave the salt (4) generated from (2) and 10,11-dihydrocinchonidine (1) as a white solid (3.13g): melting point 124.9-126°C, [α] 20 D -29.6° (c=1, MeOH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com